.gif) VIARTIS
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Parkinson's Disease News covers all significant new research, reports, books, and resources concerning Parkinson's Disease. Articles are chosen on the basis of their medical significance or potential interest. Our overwhelming priority is the facts, regardless of whether they contradict prevailing views or vested interests. Analysis and further information are provided either to explain the background or implications, or to balance misleading claims. If you notice errors or inadequacies, or dispute what is written, or want to propose articles, please e-mail [email protected].
19th January 2019 - News research ANTIEMETICS, ANTIPSYCHOTICS, ANTIDEPRESSANTS AND THE RISK OF PARKINSON'S DISEASE
Antipsychotics, antidepressants, and antiemetics are well-known causative agents of parkinsonism. However, it is not absolutely certain that the use of these medications increase the risk of Parkinson's Disease. The aim was to define the risk of Parkinson's Disease associated with use of antipsychotic, antidepressant, or antiemetic therapy. There was an observed increased risk of Parkinson's Disease with the separate use of antiemetics of 8%. There was an observed increased risk of Parkinson's Disease with the separate use of antipsychotics of 31%. There was an increased observed risk of Parkinson's Disease with the combined use of antiemetics and antipsychototics of 42%. The use of antidepressants on their own and in combination with antipsychotics was not associated with a higher risk of Parkinson's Disease. Separate or concurrent use of antiemetics and antipsychotics showed increased risks of Parkinson's Disease. Although statistical significance was observed, when taking into account that there are still unmeasured, unconsidered confounders, the level of statistical significance could be different. Caution will still be needed in prescribing these drugs in people who have Parkinson's Disease. Reference : International Journal of Clinical Pharmacology and Therapeutics [2018] 10.5414/CP203354 [Epub ahead of print] (J.Y.Shin, H.L.Jeon, H.E.Jeong, H.I.Ma, S.Jang) Complete abstract In order to refer to this article on its own click here
31st December 2018 - News report THE FDA APPROVE THEIR FIRST INHALED L-DOPA
The FDA have approved INBRIJA, which is the first and only FDA approved L-dopa inhaler for intermittent treatment of OFF episodes in people with Parkinson's Disease taking carbidopa/levodopa. It is expected to be available in the first quarter of 2019. It is based on ARCUS� Technology Platform for Inhaled Drug Delivery. For more information go to :is a new drug being developed for the treatment of Parkinson's Disease. It also includes carbidopa, which is the same as is included in Sinemet. ONO-2160 is an L-dopa pro-drug, which means that once absorbed it is converted into L-dopa. For more information go to : Inbrija The SPAN-PD trial of INBRIJA showed a statistically significant improvement in motor function after three months as measured by a reduction in Unified Parkinson's Disease Rating Scale (UPDRS) Part III score at 30 minutes. Onset of action was seen as early as 10 minutes. The most common adverse reactions with INBRIJA during the pivotal trial were cough (15% v 2% with a placebo), upper respiratory tract infection (6% v 3% with a placebo), nausea (5% v 3% with a placebo), and sputum discoloured (5% v 0% with a placebo). INBRIJA is not to be used by patients have taken a nonselective monoamine oxidase inhibitors such as phenelzine or tranylcypromine within the last two weeks. Reference : eNeurologicalScience [2018] 13 : 8-13 (M.Nomoto, M.Nagai, N.Nishikawa, R. Ando, Y.Kagamiishi, K.Yano, S.Saito, A.Takeda) Complete abstract In order to refer to this artical on its own click here
25th December 2018 - Resource COPING WITH THE COST OF CARE INCLUDING TAX DEDUCTIONS As Parkinson's Disease worsens, you may find you face more and more medical bills. No matter how great your insurance coverage is your costs can quickly get overwhelming. This web site details and advises concerning medical expenses, health aides, durable and non-duranle medical equipment, Medicaid, and medical insurance. For more information go to Advice concerning medicial expenses
30th October 2018 - New research NEW CLASSIFICATION OF PARKINSON'S DISEASE TREMOR TYPES
The reclassification of tremor types in Parkinson's Disease has been proposed according to four different types of tremor, which are as follows : Type I (resting tremor), Type II (resting tremor and action tremor with similar frequencies) divided into II-R when there is a time lag and II-C when there is no time lag, Type III (action tremor), Type IV (mixed resting and action tremor with differing frequencies) (type IV).
Mixed tremor was the most common tremor type in Parkinson's Disease in the patients tested. No significant association was found between the clinical variables and the tremor types. Reference : Parkinsons Disease [2018] [2018] : 4327597 (A.Gironell, B.Pascual-Sedano, I.Aracil, J.Mar�n-Lahoz, J.Pagonabarraga, J.Kulisevsky) Complete abstract In order to refer to this article on its own click here
18th October 2018 - New research ONO-2160 - A NEW L-DOPA PRO-DRUG FOR PARKINSON'S DISEASE
ONO-2160 is a new drug being developed for the treatment of Parkinson's Disease. It also includes carbidopa, which is the same as is included in Sinemet. ONO-2160 is an L-dopa pro-drug, which means that once absorbed it is converted into L-dopa. An open study clinical trial was carried out on people with Parkinson's Disease in which ONO-2160 and carbidopa was compared to the use of L-dopa and carbidopa, which is what is in Sinemet. Patients were primarily evaluated using the Unified Parkinson's Disease Rating Scale Part III, a Parkinson's disease symptom diary, and analysis of adverse events. Pharmacokinetic analysis of plasma levodopa concentration was also performed. ONO-2160 and carbidopa therapy stabilized the plasma concentration of L-dopa better than L-dopa with carbidopa. No adverse events with safety concerns were observed. The combination of ONO-2160 and carbidopa produced a prolonged and stable plasma L-dopa concentration whilst also reducing the Parkinson's Disease symptom scores. The combination was well tolerated with no safety concerns. The results suggest an improved method of administering L-dopa. Reference : eNeurologicalScience [2018] 13 : 8-13 (M.Nomoto, M.Nagai, N.Nishikawa, R. Ando, Y.Kagamiishi, K.Yano, S.Saito, A.Takeda) Complete abstract In order to refer to this article on its own click here
20th September 2018 - New research SARCOPENIA IS INCREASED IN PARKINSON'S DISEASE
Sarcopenia is the loss of muscle mass, strength and function related to ageing. Sarcopenia and frailty are found in up to one-third of the general elderly population. Both are associated with major adverse health outcomes such as nursing home placement, disability, decreased quality of life. For more information go to : Sarcopenia In people who have Parkinson's Disease who were in care the prevalence of sarcopenia was found to be much higher, as it was 55% and only 8% in people who did not have Parkinson's Disease. Frailty was detected in 35% of people with Parkinson's Disease. In those people with Parkinson's Disease who were not in care, 33% had sarcopenia and 22% had frailty. Both sarcopenia and frailty were significantly associated with longer disease duration of Parkinson's Disease, higher motor impairment, higher Hoehn and Yahr stages, decreased quality of life, frequency of falls, a higher non-motor symptom burden, and higher care levels. Exercise, or dietary changes, increased intake of high protein foods, or use of certain drugs can reverse sarcopenia or to prevent it from occurring. For more information go to : Sarcopenia Reference : Gerontology [2018] Sep 10 [Epub ahead of print] (M.Peball, P.Mahlknecht, M. Werkmann, K.Marini, F.Murr, H.Herzmann, H.Stockner, R.de Marzi, B.Heim, A. Djamshidian, P.Willeit, J.Willeit, S.Kiechl, D.Valent, F.Krismer, G.K.Wenning, M.Nocker, K.Mair, W.Poewe, K.Seppi) Complete abstract In order to refer to this article on its own click here
11th August 2018 - New research AMANTADINE EXTENDED-RELEASE FOR DYSKINESIA
Amantadine extended-release (ER) capsules (GOCOVRI�) are approved in the USA for the treatment of dyskinesia in people with Parkinson's Disease who are taking L-dopa. With a recommended dosage of 274 mg once daily at bedtime, the new formulation of amantadine allows a more gradual time to peak plasma amantadine concentration and higher drug concentrations in the morning and throughout the day, which is the time period when L-dopa induced dyskinesia (LID) is the most problematic. For more information go to : Gocovri
After
13 weeks and 25 weeks amantadine ER capsules significantly improved
L-dopa-induced dyskinesia (LID), while also increasing ON time without
troublesome dyskinesia and reducing OFF time and ON time with troublesome
dyskinesia from the morning and throughout the day. After 64 weeks patients
previously treated with amantadine ER maintained their improvements. Amantadine ER was generally well tolerated, with most of the adverse events (AEs) of amantadine ER being transient and mild or moderate in severity. The most common adverse events were treatment related and had an incidence of more than 15%. Reference : CNS Drugs [2018] Aug 7 [Epub ahead of print] (J.Paik, S.J.Keam) Complete abstract In order to refer to this article on its own click here
8th August 2018 - New research GARCINOL - A FUTURE MAO INHIBITOR FOR PARKINSON'S DISEASE
MAO inhibitors are often prescribed for Parkinson's Disease. MAO inhibitors help to maintain dopamine levels by inhibiting the enzyme Monoamine Oxidase (MAO). It is primarily low dopamine levels that cause Parkinson's Disease. The main MAO inhibitors in use are selegiline, rasagiline and safinamide. For more information go to : selegiline , rasagiline , safinamide . Researchers have assessed Garcinol, which originates from plant species. For more information go to : garcinol
Reference : Medical Hypotheses [2018] 117 : 54-58 (M.K.Mazumder, R.Paul, B.C.Phukan, A.Dutta, J.Chakrabarty, P.Bhattacharya, A.Borah) Complete abstract In order to refer to this article on its own click here 31st July 2018 - New research BREATH TEST FOR EARLY PARKINSON'S DISEASE
Researchers have tested a sensor to detect early Parkinson's Disease solely by using the breath of the patients. A device was previously developed that had 40 sensors based on gold nanoparticles. Each sensor had a different chemical attached to it that could bind certain volatile molecules in the breath. The device detected differences in the exhaled breath of people already being treated for Parkinson's Disease. So it was assessed whether the device could detect differences in the breath of people with early or untreated Parkinson's Disease. The sensitivity, specificity, and accuracy values of the sensor array to detect Parkinson's Disease when compared to people who did not have Parkinson's Disease controls were 79%, (sensitivity), 84% (specificity), and 81% (accuracy). The results were less than when using the more expensive Midbrain ultrasonography, which gave results of 93% (sensitivity), 90% (specificity), and 92% (accuracy). However, the results were considerably better than when using smell detection tests which gave far lesser results : 62% (sensitivity), 89% (specificity), and 73% (accuracy). So although the device needs to be improved and validated by larger studies, the researchers say that it has potential as a small, portable system to screen at-risk individuals without the need for highly trained specialists. Reference : : Journal of Neurology [2018] Jun 26 [Epub ahead of print] (A.Romagnolo, M.Fabbri, A.Merola, E.Montanaro, S.Palermo, T.Martone, A.Seresini, S.Goldwurm, M.G. Rizzone, L.Lopiano) Complete abstract In order to refer to this article on its own click here
31st July 2018 - News report ALAN ALDA DIAGNOSED WITH PARKINSON'S DISEASE Alan Alda, the 82 year old Hollywood actor, has announced that he has had Parkinson's Disease since 2015. His is a six-time Emmy Award winner, a Golden Globe Award winner, and was nominated for the Academy Award for Best Supporting Actor. He is best known for the long running tv series, the American comedy drama, M*A*S*H, and the films : The Aviator, Crimes and Misdemeanors, Manhattan Murder Mystery, and The Four Seasons. He revealed his diagnosis in 2015 in an interview with CBS This Morning, where he discussed the �full life� he�s lived since then. He decided to reveal his Parkinson's Disease diagnosis after he noticed during a TV appearance that his thumb was twitching. For more information go to the news report : News report
8th July 2018 - New research AN ASSESSMENT OF HAVING PARKINSON'S DISEASE FOR 35 YEARS
An assessment was carried out of the clinical, neuropsychological, electrophysiological, and neuroimaging features of Parkinson's Disease since the onset of motor symptoms in people who had Parkinson's Disease for over 35 years.
Even after more than 35 years of having Parkinson's Disease, L-dopa and STN-DBS remained effective to some extent, and were even more effective in combination, on the cardinal symptoms of Parkinson's Disease. Reference : Journal of Neurology [2018] Jun 26 [Epub ahead of print] (A.Romagnolo, M.Fabbri, A.Merola, E.Montanaro, S.Palermo, T.Martone, A.Seresini, S.Goldwurm, M.G. Rizzone, L.Lopiano) Complete abstract In order to refer to this article on its own click here
15th June 2018 - New research LRP10 - A NEW GENETIC FORM OF PARKINSON'S DISEASE
A new genetic form of Parkinson's Disease has been discovered called LRP10. A small proportion of cases of Parkinson's Disease have a genetic cause. Most of them make Parkinson's Disease more likely rather than inevitable. Genetic disorders normally occur due to inheritance, either autosomal recessive (from both parents) or autosomal dominant (from one parent), but can arise spontaneously. Having relatives with Parkinson's Disease does not mean that it has been inherited, because relatives can have similar environmental factors. For more information concerning genetic causes go to : Genetic causes of Parkinson's Disease
Reference : Lancet Neurology [2018] Jun 6 [Epub ahead of print] (M.Quadri, W. Mandemakers, M.M.Grochowska, R.Masius, H.Geut, E.Fabrizio, G.J.Breedveld, D. Kuipers, M.Minneboo, L.J.M.Vergouw, A.Carreras Mascaro, E.Yonova-Doing, E.Simons, T.Zhao) Complete abstract In order to refer to this article on its own click here
5th June 2018 - New research ADAPTIVE DEEP BRAIN STIMULATION FOR PARKINSON'S DISEASE
Deep Brain Stimulation is the most effective form of surgery for Parkinson's Disease. It involves the use of electrodes that are implanted into the brain and connected to a small electrical device called a pulse generator that can be externally programmed. DBS uses a surgically implanted, battery-operated medical device, which is similar to a heart pacemaker and approximately the size of a stopwatch. For more information go to : Deep Brain Stimulation
In in-clinic testing, energy savings were substantial (38%-45%), and therapeutic efficacy was maintained. This is the first demonstration of adaptive DBS in Parkinson's Disease using a fully implanted device and neural sensing. Reference : Journal of Neural Engineering [2018] 15 (4) : 046006 (N.C.Swann, C.de Hemptinne, M.C.Thompson, S.Miocinovic, A.M.Miller, R.Gilron, J.L.Ostrem, H.J.Chizeck, P.A.Starr) Complete abstract In order to refer to this article on its own click here
29th May 2018 - New clinical trial MICHAEL J.FOX FOUNDATION FUNDS ALK4290 FOR PARKINSON'S DISEASE Alkahest, a clinical-stage biotechnology company, has been awarded a grant from The Michael J.Fox Foundation for the �Development of ALK4290 as a novel PD therapeutic�. This project will focus on the mechanisms underlying immune cell involvement in Parkinson�s Disease, assessing the link between neuroinflammation and disease progression. ALK4290 is a novel, orally-available, small molecule that has been well-tolerated in previous clinical studies. They will evaluate the effect of ALK4290 in two pre-clinical models relevant to Parkinson's Disease biology. A toxin-induced and a transgenic model of disease will allow them to assess the efficacy of the compound on Parkinson's Disease-relevant processes. For more information go to Alkahest
24th May 2018 - New cliniacl trial DEMENTIA DRUG FOR REDUCING FALLS IN PARKINSON'S DISEASE
A
UK-wide trial into Parkinson�s Disease led by Royal United Hospitals Bath
NHS Foundation Trust and the University of Bristol will assess whether a
commonly prescribed dementia drug
(a cholinesterase inhibitor)
could prevent debilitating falls for people with Parkinson's Disease. Falls
are a frequent complication in people with Parkinson�s Disease. Earlier
phase trials which showed that cholinesterase inhibitors have the potential
to almost halve the number of falls in people with Parkinson's Disease and
improve the regularity of their walking, speed, and balance.
For more information go to
University
of Bristol
11th May 2018 - New research METOCLOPRAMIDE INCREASES THE RISK OF PARKINSON'S DISEASE Metoclopramide is Dopamine D2 receptor antagonist. Dopamine antagonists inhibit the dopamine receptors. Metoclopramide is a drug used mostly for stomach and esophageal problems. It increases muscle contractions in the upper digestive tract. This speeds up the rate at which the stomach empties into the intestines. For more information go to Metoclopramide The risk of Parkinson's Disease is increased 1.73 times in standard exposure users of metoclopramide, and 3.15 times in high exposure users. The average increase in risk of Parkinson's Disease was 2.16 times normal. That means that the increased risk is still low. However, since long-duration exposure accounts for the majority of users and parkinsonism in the high-exposure group, physicians are advised to avoid prescribing metoclopramide for morer than 5 days, even if the daily dose is 30 mg. Reference : British Journal of Clinical Pharmacology [2018] May 10 [Epub ahead of print] (S.C.Tsai, S.Y.Sheu, L.N.Chien, H.C.Lee, E.J.Yuan, R.Y.Yuan) Complete abstract
25th April 2018 - New research MICROTABLET DISPENSER FOR MORE PRECISE L-DOPA DOSAGE
A study has assessed the effect of introducing a microtablet dose dispenser and adjusting doses based on the monitoring of motor symptoms in people with Parkinson's Disease. Forms of L-dopa are usually limited to certain doses and are consequently not adaptable to needs. A microtablet dose dispenser enables more precise doses of L-dopa. Daytime doses of L-dopa were replaced with L-dopa / carbidopa microtablets, 5mg / 1.25 mg delivered from a dose dispenser device with programmable reminders. After two weeks, doses were adjusted based on ambulatory accelerometry and clinical monitoring. The daily L-dopa dose was increased by 15% from period 1 to 2, and the dose interval was reduced by 12%. The MDS-UPDRS parts II and III, disease-specific quality of life (PDQ-8), wearing-off symptoms (WOQ-19), and nonmotor symptoms improved after dose increases. Evaluation of accelerometry results demonstrated improvement in 60% of subjects and worsening in 25% of them. The introduction of an L-dopa microtablet dispenser and accelerometry aided dose adjustments Parkinson's Disease improve symptoms and quality of life in the short term. Reference : CNS Neuroscience and Therapeutics [2018] 24 (5) : 439-447 (D.Jan Johansson, A.Ericsson, A.Johansson, A.Medvedev, D.Nyholm, F.Ohlsson, M.Senek, J.Spira, I.Thomas, J.Westin, F.Bergquist) Complete abstract In order to refer to this article on its own click here
20th April 2018 - New research MILD BRAIN INJURY INCREASES RISK OF PARKINSON'S DISEASE
20th March 2018 - New research EXTENDED RELEASE AMANTADINE FOR DYSKINESIA IN PARKINSON'S DISEASE
24th February 2018 - New research THE RETINA DETERIORATES IN PARKINSON'S DISEASE
A single group of dopaminergic neurons (A17) has been found in the retina, which is why it is affected in Parkinson's Disease. Patterns of RNFL (retinal nerve fibre layer) and macular damage detected by OCT could consequently be a useful biomarker for evaluating the progression of PD. Reference : Journal of Parkinsons Disease [2018] 8 (1) : 85-92 (L.J.Ma, L.L.Xu, C.J.Mao, Y.T.Fu, X.Y.Ji, Y.Shen, J.Chen, Y.P.Yang, C.F.Liu) Complete abstract
28th January 2018 - New research LONG TERM EFFECTS OF RASAGILINE ON PARKINSON'S DISEASE
Reference : Movement Disorders [2016] 31 (10) : 1489-1496 -274 (O.Rascol, R.A.Hauser, F.Stocchi, C.J.Fitzer-Attas, Y.Sidi, V.Abler, C.W.Olanow) Complete abstract In order to refer to this article on its own click here
25th January 2018 - New research HIGH LEVELS OF VITAMIN B6 INTERFERE WITH L-DOPA IN PARKINSON'S DISEASE
Reference : Shokuhin Eiseigaku Zasshi [2017] 58 (6) : 268-274 (Y.Sato, C.Yasumiishi, T.Chiba, K.Umegaki) Complete abstract In order to refer to this article on its own click here
23rd January 2018 - New research ALPHA-SYNUCLEIN DOES NOT INDICATE PARKINSON'S DISEASE
Although it is sometimes claimed that an accumulation of alpha-synuclein causes Parkinson's Disease, Parkinson's Disease instead can cause alpha-synuclein to accumulate. It has been suggested that alpha-synuclein could therefore be a valuable biomarker for Parkinson's Disease. So the levels of alpha-synuclein in the plasma was assessed in various types of Parkinson's Disease. Alpha-synuclein is commonly found in the brain. For more information go to : Alpha-synuclein
Results : Levels of alpha-synuclein did not differ significantly between people with Parkinson's Disease and people who did not have Parkinson's Disease. There was no difference between different subtypes. Yet there was an inverse correlation between alpha-synuclein and the Postural Instability and Gait Disorder (PIGD) subtype. Conclusions : Plasma alpha-synuclein levels are not valuable biomarkers of Parkinson's Disease. It does not differ in the subtypes of Parkinson's Disease either. However, there is an inverse relation between plasma alpha-synuclein levels level and the severity of Parkinson's Disease in the Postural Instability and Gait Disorder (PIGD) subtype. Reference : Neurologia i Neurochirurgia Polska [2017] Nov 21 [Epub ahead of print] (M. Malec-Litwinowicz, A.Plewka, D.Plewka, E.Bogunia, M.Morek, A.Szczudlik, M. Szubiga, M.Rudzinska-Bar) Complete abstract In order to refer to this article on its own click here
12th January 2018 - New research SEXUAL DYSFUNCTION IN WOMEN WITH PARKINSON'S DISEASE
In women with Parkinson's Disease there can be a profound impairment in sexual arousal, behaviour, orgasm, sexual satisfaction and sex drive. The objectives of this study were to assess the level of sexual function in women with Parkinson's Disease, and the description of sexual dysfunctions.
Sexual dysfunction was found to be more prevalent in women with Parkinson's Disease and is predicted by older age and the severity of depressive symptoms. Reference : Movement Disorders [2016] 31 (11) : 1685-1693 (S.Varanda, J.Ribeiro da Silva, A.S.Costa, C.Amorim de Carvalho, J.N.Alves, M.Rodrigues, G.Carneiro) Complete abstract In order to refer to this article on its own click here
6th January 2018 - New research L-DOPA INHALER RAPIDLY IMPROVES PARKINSON'S DISEASE
Background : The L-dopa inhaler CVT-301 is presently being assessed for its use in Parkinson's Disease. It is designed to deliver a precise dose of a dry powder formulation of L-dopa. Inhaled L-dopa enters the body through the lungs and then reaches the brain far more quickly by bypassing the digestive system. For more information go to : CVT-301
Conclusion : As the effect was already seen after 10 minutes, which is the shortest time assessed, the effect could be even quicker. Consequently, CVT-301 could become of widespread use in Parkinson's Disease for when a quick effect is needed. Reference : Movement Disorders [2016] 31 (9) : 1356-1365 (P.A.LeWitt, R.A.Hauser, D.G. Grosset, F.Stocchi, M.H.Saint-Hilaire, A.Ellenbogen, M.Leinonen, N.B.Hampson, T.DeFeo-Fraulini, M.I.Freed, K.D.Kieburtz) Complete abstract In order to refer to this article on its own click here
5th January 2018 - New research CAFFEINE LEVELS CAN INDICATE EARLY PARKINSON'S DISEASE
Reference : Neurology [2018] Jan 3 [Epub ahead of print] (M.Fujimaki, S.Saiki, Y.Li, N.Kaga, H.Taka, T.Hatano, K.I.Ishikawa, Y.Oji, A.Mori, A.Okuzumi, T.Koinuma, S.I.Ueno, Y.Imamachi, T.Ueno, Y.Miura, M.Funayama, N.Hattori) Complete abstract
31st December 2017 - New research CAMICINAL INCREASES L-DOPA LEVELS IN PARKINSON'S DISEASE
Delayed gastric emptying can impair absorption of L-dopa, thereby contributing to motor fluctuations. Camicinal (GSK962040), is a gastroprokinetic, which is being assessed for its effect on the absorption of L-dopa and the symptoms of Parkinson's Disease. Gastroprokinetic drugs increase the movement of ingested material through the GI tract.
Camicinal resulted in significant reduction in OFF time, reducing it by 2 hours 18 minutes. There was a significant increase in ON time, increasing it by 1 hour 52 minutes. There was also a significant decrease in mean total MDS-UPDRS score (Parkinson's Disease symptoms). Camicinal treatment was generally well tolerated. Parkinson's Disease symptom improvement with the use of camicinal occurred in parallel with a more rapid absorption of L-dopa. This study provides evidence of an improvement of the motor response to L-dopa in people with Parkinson's Disease treated with camicinal Reference : Movement Disorders [2017] Dec 26 [Epub ahead of print] (S.L.Marrinan, T.Otiker, L.S.Vasist, R.A.Gibson, B.K.Sarai, M.E.Barton, D.B.Richards, P.M.Hellstr�m, D.Nyholm, G.E.Dukes, D.J.Burn) Complete abstract In order to refer to this article on its own click here
27th December 2017 - New research ORALLY DISSOLVING FILM FOR TAKING ROPINIROLE
Ropinirole is a dopamine agonist used for Parkinson's Disease. It is sold as Requip, Repreve, Ronirol, and Adartrel. For more information go to For more information go to : Requip However, ropinirole undergoes extensive metabolism, resulting in a low oral bioavailability. The necessity of frequent administration due to the short half-life of ropinirole may also lessen patient compliance. There is consequently a pressing need to devise formulations for the delivery of ropinirole that allow simple and easy administration.
In conclusion, the ropinirole oral film improved bioavailability after sublingual or buccal administration. This formulation potentially overcomes biopharmaceutical challenges and provides a convenient means of administration of ropinirole and also other Parkinson's Disease drugs. Reference : Colloids and Surfaces, B Biointerfaces [2017] 163 : 9-18 [Epub ahead of print] (K.L.Lai, Y.Fang, H.Han, Q.Li, S.Zhang, H.Y.Li, S.F.Chow, T.N.Lam, W.Y.T.Lee) Complete abstract In order to refer to this article on its own click here
22nd December 2017 - New research LASER SHOES FOR PARKINSON'S DISEASE
Laser shoes have been developed to help with the freezing that can occur in Parkinson's Disease. When freezing (freezing of gait) occurs, the person finds themself unable to take a step despite willing themselves to walk forward. This can occur for anything from a few seconds to over a minute and can cause a person to lose their balance and fall over. Each laser shoe has a projection device mounted to the toe that produces a line about 18 inches (half a metre) ahead, towards which the user can then step. Patients with Parkinson's Disease who had freezing of gait were assessed in "off" and also "on" medication states.
These findings demonstrate the efficacy of laser shoes and so offer a promising potential to deliver cueing for people with freezing of gait. Reference : Neurology [2017] Dec 20 [Epub ahead of print] (C.Barthel, J.Nonnekes, M.van Helvert, R.Haan, A.Janssen, A.Delval, V.Weerdesteyn, B.Deb�, R.van Wezel, B.R.Bloem, M.U.Ferraye) Complete abstract In order to refer to this article on its own click here
12th December 2017 - New research EARLY WEIGHT LOSS IN PARKINSON'S DISEASE
Weight changes over time in people with Parkinson's Disease and in those with atypical parkinsonism and can be associated with an increased risk of dependency, dementia and death.
Reference : Neurology [2017] 89 (22) : 2254-2261 (K.Cumming, A.D.Macleod, P.K.Myint, C.E.Counsell) Complete abstract In order to refer to this article on its own click here
30th November 2017 - New research EFFICACY AND SAFETY OF PERAMPANEL IN PARKINSON'S DISEASE
Reference : British Journal of Pharmacology [2017] Jul 1 [Epub ahead of print] (D.Lindenbach, B.Das, M.Conti, S.M.Meadows, A.K.Dutta, C.Bishop) Complete abstract
27th November 2017 - New research BLADDER AND BOWEL SYMPTOMS IN PARKINSON'S DISEASE
An assessment was made of the prevalence of urinary and bowel symptoms, such as urinary incontinence and constipation in people with early Parkinson's Disease. Prevalent bladder symptoms (urinary incontinence and nighttime voiding) and bowel symptoms (constipation and fecal incontinence) were defined as occurring at least sometimes.
Reference : Neurourology and Urodynamics 2017 Nov 2 [Epub ahead of print] (M.C.Serra, A.Landry, J.L.Juncos, A.D.Markland, K.L.Burgio, P.S.Goode, T.M.Johnson, C.P.Vaughan) Complete abstract In order to refer to this article on its own click here
18th November 2017 - News report JESSE JACKSON DIAGNOSED WITH PARKINSON'S DISEASE
28th October 2017 - New research THE EFFECT OF MEDICAL CANNABIS ON PARKINSON'S DISEASE
Medical cannabis or medical marijuana, are cannabis or marijuana that is recommended by doctors for their patients. It has been claimed to be beneficial for people with Parkinson's Disease. However, its use is controversial, because support for its benefits is based on small clinical studies. For more information go to : Medical cannabis
Reference : Clinical Neuropharmacology [2017] Oct 20 [Epub ahead of print] (Y.Balash, L.Bar-Lev Schleider, A.D.Korczyn, H.Shabtai, J.Knaani, A.Rosenberg, Y.Baruch, R. Djaldetti, N.Giladi, T.Gurevich) Complete abstract In order to refer to this article on its own click here
23rd October 2017 - New research PASSIVE SMOKING INCREASES THE RISK OF PARKINSON'S DISEASE
Tobacco smoking is consistently associated with a reduced likelihood of Parkinson's Disease in men and women. So passive smokers were assessed to see whether they were affected in the same way.
However, household exposure during adulthood was associated with a reduced risk of Parkinson's Disease down to 59% of what would be expected. Household exposure during childhood was not associated with any alteration in the risk of Parkinson's Disease. Workplace exposure was also not associated with the risk of Parkinson's Disease. Among lifelong non-smokers, passive smoke exposure combined across all settings and accumulated over a lifetime was only marginally associated with an increased risk of Parkinson's Disease. Reference : Parkinsonism and Related Disorders [2017] Oct 4 [Epub ahead of print] (N.M. Gatto, D.Deapen, Y.Bordelon, S.Marshall, L.Bernstein, B.Ritz) Complete abstract In order to refer to this article on its own click here
18th October 2017 - New research DRIVING SAFETY DECLINES IN PARKINSON'S DISEASE
People with Parkinson's Disease have been assumed to decline in their driving ability due to the problems that Parkinson's Disease causes. People with Parkinson's Disease were assessed in their driving ability and again two years later.
However, two years later, people with Parkinson's Disease showed greater cognitive decline and worse visual acuity. They had a greater increase in driving errors, with an increase in errors over 4 times that of people who did not have Parkinson's Disease. So it appears that initially although driving ability does not greatly worsen in people with Parkinson's Disease, that driving ability gradually worsens over time. Reference : Neurology [2017] Oct 11 [Epub ahead of print] (E.Y.Uc, M.Rizzo, A.M.J. O'Shea, S.W.Anderson, J.D.Dawson) Complete abstract In order to refer to this article on its own click here
30th September 2017 - New research ALPHA-SYNUCLEIN PREDICTS THE COGNITIVE DECLINE IN PARKINSON'S DISEASE
It is often claimed that alpha-synculein is a hallmark of Parkinson's Disease or indicates its severity. It has even been wrongly claimed to be the primary cause of Parkinson's Disease. However, it has instead been found to be related to the cognitive decline that can occur in Parkinson's Disease, especially as Parkinson's Disease worsens.
The results suggest that plasma alpha-synuclein level correlates with cognitive decline but not with motor severity in people with Parkinson's Disease. Plasma alpha-synuclein could therefore serve as a biomarker for people with Parkinson's Disead at risk of cognitive decline. Reference : Journal of Neurology, Neurosurgery and Psychiatry [2017] 88 (10) : 818-824 (C.H.Lin, S.Y.Yang, H.E.Horng, C.C.Yang, J.J.Chieh, H.H.Chen, B.H.Liu, M.J.Chiu) Complete abstract In order to refer to this article on its own click here
2nd September 2017 - New research ASTHMATIC INHALER FOR PARKINSON'S DISEASE
Reference : Science [2017] 357 (6354) : 891-898 (S.Mittal, K.Bj�rnevik, D.S.Im, A.Flierl, X.Dong, J.J.Locascio, K.M.Abo, E.Long, M.Jin, B.Xu, Y.K.Xiang, J.C.Rochet, A.Engeland, P.Rizzu, P.Heutink, T.Bartels, D.J.Selkoe, B.J.Caldarone, M.A.Glicksman, V.Khurana, B.Sch�le, D.S.Park, T.Riise, C.R.Scherzer) Complete abstract
26th August 2017 - News release GOCOVRI� APPROVED FOR DYSKINESIA IN PARKINSON'S DISEASE Adamas Pharmaceuticals announced that the U.S. Food and Drug Administratio (FDA) has approved GOCOVRI (274mg amantadine) extended release capsules (previously ADS-5102) for the treatment of dyskinesia in people with Parkinson's Disease receiving L-dopa based therapy, with or without additional dopaminergic medications.
19th August 2017 - New research FAILED FACIAL EXPRESSION IN PARKINSON'S DISEASE
People with Parkinson's Disease commonly have impairment of facial expressivity (hypomimia) and also have difficulties in interpreting the emotional facial expressions of other people, especially for aversive emotions. The ability of recognising emotional facial expressions by people with Parkinson's Disease was assessed using the Ekman 60-faces test (Emotion recognition task).
The correlation between the recognition of emotions and the expression of emotions suggests that they share a common cause, which could be deteriorated in people with Parkinson's Disease. Reference : PLoS One [2017] 12 (1) : e0169110 (L.Ricciardi, F.Visco-Comandini, R.Erro, F.Morgante, M.Bologna, A.Fasano, D.Ricciardi, M.J.Edwards, J.Kilner) Complete abstract In order to refer to this article on its own click here
13th August 2017 - New research OCCUPATIONAL PESTICIDE USE IN PARKINSON'S DISEASE
Researchers assessed the influence of occupational pesticide use on the prevalence of Parkinson's Disease in people with information available concerning occupational, residential, and household sources of pesticide exposure.
Most surprisingly, the researchers estimated higher risks of Parkinson's Disease among those people reporting use of personal protective equipment (PPE). This suggests that personal protective equipment is insufficient for protection against pesticides. Reference : Environment International [2017] Aug 2 [Epub ahead of print] (S.Narayan, Z.Liew, J.M.Bronstein, B.Ritz) Complete abstract In order to refer to this article on its own click here
8th August 2017 - New book THE ENLIGHTENED MR. PARKINSON : THE PIONEERING LIFE OF A FORGOTTEN SURGEON Cherry Lewis
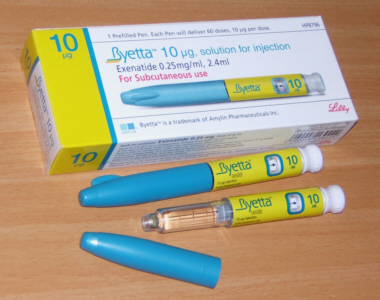
4th August 2017 - New research DIABETIC DRUG ASSESSED FOR USE IN PARKINSON'S DISEASE
Exenatide is a type 2 diabetes treatment that differs in its pharmacological action and structure from insulin. Exenatide is an injected glucagon-like peptide-1 agonist. The possible means of how it might affect Parkinson's Disease is not known. It has no direct effect on dopamine.
In four previous studies the efficacy was also found to be mild. Unusually, the effects of exenatide on Parkinson's Disease had continued to some extent beyond its use. In those studies exenatide was well tolerated but weight loss was common. Other adverse effects of the use of exenatide were nausea, injection-site induration, dyslipidemia, vomiting, diarrhoea, headache and hypoglycaemia.
Reference :
The Lancet [2017] S0140-6736(17)31585-4390 (D.Athauda, K.Maclagan,
S.S.Skene, M.Bajwa-Joseph, D.Letchford, K.Chowdhury, S.Hibbert, N.Budnik,
L.Zampedri, J.Dickson, Y.Li, I.Aviles-Olmos, T.T.Warner, P.Limousin,
A.J.Lees, N.H.Greig, S.Tebbs, T.Foltynie)
Complete
abstract
In order to refer to this article on its own
click here
1st August 2017 - New research NEW DOPAMINE AGONIST BEING ASSESSED FOR PARKINSON'S DISEASE
Reference : British Journal of Pharmacology [2017] Jul 1 [Epub ahead of print] (D.Lindenbach, B.Das, M.Conti, S.M.Meadows, A.K.Dutta, C.Bishop) Complete abstract
29th July 2017 - News release CLINICAL TRIAL OF FOLIGLURAX FOR PARKINSON'S DISEASE Prexton Therapeutics have announced the launch of phase II clinical testing of Foliglurax in people with Parkinson�s Disease. The results of a phase I clinical trial showed that Foliglurax was safe and well-tolerated.
25th July 2017 - New research CANNABIS USE IN PARKINSON'S DISEASE
Reference : Complementary Therapies in Medicine [2017] 33 : 99-104 (J.H.Kindred, K.Li, N.B.Ketelhut, F.Proessl, B.W.Fling, J.M.Honce, W.R.Shaffer, T.Rudroff) Complete abstract
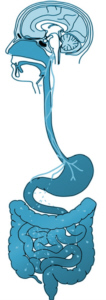
21st July 2017 - New research THE GUT BRAIN AXIS IN PARKINSON'S DISEASE
22nd June 2017 - New research AUTOIMMUNITY AND PARKINSON'S DISEASE
12th June 2017 - News release CDNF CLINICAL TRIAL FOR PARKINSON'S DISEASE
7th June 2017 - New research WIRELESS DEEP BRAIN STIMULATION FOR PARKINSON'S DISEASE
Deep brain stimulation (DBS) is the most commonly performed and most effective surgery for Parkinson's Disease but is unaffordable to a lot of people. It involves the use of electrodes implanted into the brain connected to an electrical device. For more information go to : Deep Brain Stimulation A novel wireless method of administering brain stimulation has been introduced that requires no implants or external connections. By injecting magnetic nanoparticles into the brain, neurons can be manipulated by applying external magnetic fields. These particles are capable of deep penetration of brain tissue and can stimulate nerve cells. For more information go to : Wireless Deep Brain Stimulation
Reference : Stereotactic and Functional Neurosurgery [2017] 95 (3) : 174-182 [Epub ahead of print] (D.Li, C.Zhang, J.Gault, W.Wang, J.Liu, M.Shao, Y.Zhao, K.Zeljic, G.Gao, B.Sun) Complete abstract In order to refer to this article on its own click here
4th June 2017 - New research AIR POLLUTION INCREASES THE RISK OF PARKINSON'S DISEASE
Researchers investigated the effects of air pollution on the risk of Parkinson's Disease. They primarily assessed atmospheric particulate matter (PM), which are solid or liquid matter that is suspended in the atmosphere. Sources of particulate matter can be man-made or natural, and can very adversely affect human health. It includes particles such as dust, pollen, soot, smoke, and liquid droplets. Also assessed was nitrogen dioxide (NO2), which is a prominent air pollutant. Nitrogen dioxide is formed during the industrial synthesis of nitric acid, millions of tons of which are produced each year.
Reference : Environmental Health Perspectives [2016] 124 (11) : 1759-1765 (R.Liu, M.T. Young, J.C.Chen, J.D.Kaufman, H.Chen) Complete abstract In order to refer to this article on its own click here
29th May 2017 - New research CLINICAL TRIAL OF SAFINAMIDE FOR PARKINSON'S DISEASE
Safinamide has both dopaminergic properties (highly selective and reversible inhibition of monoamine oxidase-B) and non-dopaminergic properties (selective sodium channel blockade and calcium channel modulation, with consequent inhibition of excessive glutamate release). It is usually added to the use of L-dopa or dopamine agonists. For more information go to Xadago : Xadago
Reference : JAMA Neurology [2017] 74 (2) : 216-224 (A.H.Schapira, S.H.Fox, R.A.Hauser, J.Jankovic, W.H.Jost, C.Kenney, J.Kulisevsky, R.Pahwa, W.Poewe, R.Anand) Complete abstract
17th May 2017 - New research LIQUID L-DOPA FOR PARKINSON'S DISEASE
Liquid L-dopa is usually taken as a combination of L-dopa, carbidopa, and ascorbic acid (vitamin C) in a solution called LCAS. Therapy with liquid L-dopa has been used in people with advanced Parkinson's Disease for many years. However, long-term follow-up information is scarce. The present study aimed to determine the long-term retention rate for LCAS therapy, and to identify the causes of LCAS therapy withdrawal.
Reference : Journal of Neurological Science [2017] 377 : 6-11 (H.J.Yang, G.Ehm, Y.E.Kim, J.Y.Yun, W.W.Lee, A.Kim, H.J.Kim, B.Jeon) Complete abstract
16th April 2017 - New research PREVALENT ESOPHAGEAL SYMPTOMS IN PARKINSON'S DISEASE
Dysphagia (difficulty in swallowing) is a common problem in people with Parkinson's Disease. In order to assess the prevalence of dysphagia and other related symptoms, people with Parkinson's Disease presenting with dysphagia, odynophagia, heartburn, regurgitation, chest pain, and weight loss underwent evaluation using high-resolution manometry (HRM).
Reference : Diseases of the Esophagus [2017] 30 (4) : 1-6 (A.Su, R.Gandhy, C.Barlow, G.Triadafilopoulos) Nature Biotechnology [2017] Apr 10 [Epub ahead of print] Complete abstract
12th April 2017 - New research DOPAMINERGIC NEURONS MADE FROM ASTROCYTES
Dopaminergic neurons, the cells involved in Parkinson's Disease, have been directly converted from astrocytes, which are a completely different type of cell. The source of the cells were humans, and mice who did not have Parkinson's Disease. This is different from previous methods, which have aimed at transplanting the cells affected by the pathological process. The aim of the treatment is to eventually treat Parkinson's Disease.
Reference : Nature Biotechnology [2017] Apr 10 [Epub ahead of print] (P.R.di Val Cervo, R.A.Romanov, G.Spigolon, D.Masini, E.Mart�n-Monta�ez, E.M.Toledo, G.La Manno, M.Feyder, C.Pifl, Y.H.Ng, S.P.S�nchez, S.Linnarsson, M.Wernig, T.Harkany, G.Fisone, E.Arenas) Complete abstract
11th April 2017 WORLD PARKINSON'S DISEASE DAY
The 11th April every year is designated as World Parkinson's Disease Day. The aim of World Parkinson's Disease Day is to raise public awareness of Parkinson's Disease, a medical disorder that begins mildly but that can end up having serious and widespread effects that affect every system in the body.
9th April 2017 - New research DEXTROMETHORPHAN FOR PARKINSON'S DISEASE DYSKINESIA
Dextromethorphan/quinidine, whose trade name Nuedexta, is a combination drug containing dextromethorphan and the antiarrhythmic agent quinidine. Quinidine is included to inhibit dextromeththorphan metabolism. For more information go to Nuedexta This pilot-study (NCT01767129) examined the safety and efficacy of dextromethorphan plus quinidine for treating L-dopa induced dyskinesia. People with Parkinson's Disease were randomised to 45mg dextromethorphan and 10mg twice daily, alternated with a placebo.
Reference : Movement Disorders [2017] Mar 30 [Epub ahead of print] (S.H.Fox, L.V. Metman, J.G.Nutt, M.Brodsky, S.A.Factor, A.E.Lang, L.E.Pope, N.Knowles, J.Siffert) Complete abstract In order to refer to this article on its own click here
7th April 2017 - New research P2B 001 (RASAGILINE AND PRAMIPEXOLE) FOR PARKINSON'S DISEASE
P2B 001 is a novel combination of (1) slow release and (2) low dose rasagiline and pramipexole for synergistic use in early Parkinson's Disease that is presently in development. For more information go to P2B 001 Rasagiline is a MAO inhibitor for use in treating Parkinson's Disease. For more information go to Rasagiline Pramipexole is a dopamine agonist that is also for use in Parkinson's Disease. For more information go to : Pramipexole
Reference : Movement Disorders [2017] Apr 3 [Epub ahead of print] (C.W.Olanow, K. Kieburtz, M.Leinonen, L.Elmer, N.Giladi, R.A.Hauser, O.S.Klepiskaya, D.L.Kreitzman, M. F.Lew, D.S.Russell, S.Kadosh, P.Litman, H.Friedman, N.Linvah) Complete abstract In order to refer to this article on its own click here
4th April 2017 - New research POSTURAL DEFORMITIES IN PARKINSON'S DISEASE
Striatal (hand and foot) and postural deformities are known to commonly occur in people with atypical Parkinsonism, but also occur in people with Parkinson's Disease. These deformities are frequently misdiagnosed as joint or orthopaedic problems that can often lead to unnecessary investigations. For more information go to : Postural deformities
The results showed that striatal and postural deformities were common and present in about half of the people with Parkinson's Disease. These deformities we more common in people in the advanced stages of Parkinson's Disease. Reference : Indian Journal of Medical Research [2016] 144 (5) : 682-688 (S.Pandey, H.Kumar) Complete abstract In order to refer to this article on its own click here
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
.gif) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| �2006-2017 Viartis | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| [email protected] | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||










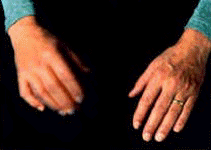 Patients
were assessed for their type of tremor. Variables were assessed including
age at diagnosis, disease duration, and UPDRS (Parkinson's Disease) scores
for rigidity. The Parkinson's Disease patients studied had the following
prevalence of tremor types as follows : Type I (resting tremor) 30%, Type II
(resting tremor and action tremor with similar frequencies) 50% divided into
II-R (25%) when there is a time lag and II-C (25%) when there is no time
lag, Type III (action tremor) 19%, Type IV (mixed resting and action tremor
with differing frequencies) 1%.
Patients
were assessed for their type of tremor. Variables were assessed including
age at diagnosis, disease duration, and UPDRS (Parkinson's Disease) scores
for rigidity. The Parkinson's Disease patients studied had the following
prevalence of tremor types as follows : Type I (resting tremor) 30%, Type II
(resting tremor and action tremor with similar frequencies) 50% divided into
II-R (25%) when there is a time lag and II-C (25%) when there is no time
lag, Type III (action tremor) 19%, Type IV (mixed resting and action tremor
with differing frequencies) 1%. 
 The
patients who were assessed had been treated with L-dopa and subthalamic
nucleus deep brain stimulation (STN-DBS). Despite the amount of time that
had elapsed there was still a sustained motor response to L-dopa (range
14%-35%), a sustained response to STN-DBS (range 23%-38%), and also to
L-dopa plus STN-DBS (37%-63%). There were mild to moderate non-motor
symptoms (within a range of 19 to 83 on a scale of 0 to 360), and autonomic
dysfunction (within a range of 8-28 on a scale of 0-69).
Two patients were demented, one had mild cognitive impairment, and two were
cognitively preserved. Three of the patients had a peripheral neuropathy and
two had a moderate-to-severe autonomic neuropathy. Their genetic tests were
unremarkable.
The
patients who were assessed had been treated with L-dopa and subthalamic
nucleus deep brain stimulation (STN-DBS). Despite the amount of time that
had elapsed there was still a sustained motor response to L-dopa (range
14%-35%), a sustained response to STN-DBS (range 23%-38%), and also to
L-dopa plus STN-DBS (37%-63%). There were mild to moderate non-motor
symptoms (within a range of 19 to 83 on a scale of 0 to 360), and autonomic
dysfunction (within a range of 8-28 on a scale of 0-69).
Two patients were demented, one had mild cognitive impairment, and two were
cognitively preserved. Three of the patients had a peripheral neuropathy and
two had a moderate-to-severe autonomic neuropathy. Their genetic tests were
unremarkable. The
gene, which is called LRP10, is on Chromosome 14. The type of inheritance is
unknown. This genetic form of Parkinson's Disease was found in Italian
individuals and somebody from Taiwan but that does not mean that other
nationalities are not affected. Of those people that had rare and
potentially pathogenic variants, four of them had Parkinson's Disease, two
of them had Parkinson's Disease with dementia, and two of them had
Parkinson's Disease with dementia and Lewy bodies. The findings implicate
LRP10 gene defects in the development of inherited forms of
a-synucleinopathies. Being newly discovered there are no genetic tests for
LRP10.
The
gene, which is called LRP10, is on Chromosome 14. The type of inheritance is
unknown. This genetic form of Parkinson's Disease was found in Italian
individuals and somebody from Taiwan but that does not mean that other
nationalities are not affected. Of those people that had rare and
potentially pathogenic variants, four of them had Parkinson's Disease, two
of them had Parkinson's Disease with dementia, and two of them had
Parkinson's Disease with dementia and Lewy bodies. The findings implicate
LRP10 gene defects in the development of inherited forms of
a-synucleinopathies. Being newly discovered there are no genetic tests for
LRP10.
 Rasagiline
is a MAO inhibitor, sold as Azilect, which is used for the treatment of
Parkinson's Disease. For more information go to
Rasagiline
is a MAO inhibitor, sold as Azilect, which is used for the treatment of
Parkinson's Disease. For more information go to








 Salbutamol,
which is normally used in inhalers for asthma, was found to reduce the risk
of Parkinson's Disease by a third. Those people taking higher doses were
half as likely to develop Parkinson's Disease. Salbutamol is
a β2-adrenoreceptor (β2AR) agonist, which
is also a regulator of the α-synuclein gene (SNCA). Alpha-synuclein
can accumulate in Parkinson's Disease. Consequently, it has been suggested
that salbutamol reduces the risk of Parkinson's Disease by lowering the
levels of α-synuclein. However, α-synuclein does not cause Parkinson's
Disease. Parkinson's Disease that can cause an accumulation of
α-synuclein. The effect of Salbutamol on Parkinson's Disease may
be due to β2-adrenoreceptor (β2AR) agonists also having an anti-cholinergic
effect. Anti-cholinergics are used for Parkinson's Disease.
Salbutamol,
which is normally used in inhalers for asthma, was found to reduce the risk
of Parkinson's Disease by a third. Those people taking higher doses were
half as likely to develop Parkinson's Disease. Salbutamol is
a β2-adrenoreceptor (β2AR) agonist, which
is also a regulator of the α-synuclein gene (SNCA). Alpha-synuclein
can accumulate in Parkinson's Disease. Consequently, it has been suggested
that salbutamol reduces the risk of Parkinson's Disease by lowering the
levels of α-synuclein. However, α-synuclein does not cause Parkinson's
Disease. Parkinson's Disease that can cause an accumulation of
α-synuclein. The effect of Salbutamol on Parkinson's Disease may
be due to β2-adrenoreceptor (β2AR) agonists also having an anti-cholinergic
effect. Anti-cholinergics are used for Parkinson's Disease. GOCOVRI
is expected to be available in the fourth quarter, and be formally launched
with the Adamas's sales force in January 2018. In clinical studies
GOCOVRI
is expected to be available in the fourth quarter, and be formally launched
with the Adamas's sales force in January 2018. In clinical studies
 For
emotion recognition, people with Parkinson's Disease reported lower scores than
average. There was a particular difficulty by people with Parkinson's
Disease in recognising certain emotions including happiness, fear, anger,
sadness and surprise. With showing facial emotions people with Parkinson's
Disease differed from normal, especially regarding happiness, sadness,
and anger, which were displayed by them less than normal. There was a relationship
between emotion
facial recognition and facial expression in people with or without
Parkinson's Disease. It appears they go together.
For
emotion recognition, people with Parkinson's Disease reported lower scores than
average. There was a particular difficulty by people with Parkinson's
Disease in recognising certain emotions including happiness, fear, anger,
sadness and surprise. With showing facial emotions people with Parkinson's
Disease differed from normal, especially regarding happiness, sadness,
and anger, which were displayed by them less than normal. There was a relationship
between emotion
facial recognition and facial expression in people with or without
Parkinson's Disease. It appears they go together.  Ever
having used carbamate pesticides increased the risk of Parkinson's Disease
by 455%, while the use of organophosphorus pesticides (OP) and
organochlorine pesticides (OC) doubled the risk of Parkinson's Disease.
The risk of developing Parkinson's Disease increased by 110% to 211% if somebody had ever had
occupational use of fungicides, herbicides, and insecticides. Using any
pesticide occupationally for more than 10 years doubled the risk of
Parkinson's Disease compared with those people that had no occupational
pesticide use.
Ever
having used carbamate pesticides increased the risk of Parkinson's Disease
by 455%, while the use of organophosphorus pesticides (OP) and
organochlorine pesticides (OC) doubled the risk of Parkinson's Disease.
The risk of developing Parkinson's Disease increased by 110% to 211% if somebody had ever had
occupational use of fungicides, herbicides, and insecticides. Using any
pesticide occupationally for more than 10 years doubled the risk of
Parkinson's Disease compared with those people that had no occupational
pesticide use.  Publisher's
description : In 1817 - two hundred years years ago - James Parkinson
(1755�1824) defined this mysterious ailment so precisely that we still diagnose
Parkinson's Disease today by recognizing the symptoms he identified. The story
of this remarkable man�s contributions to the Age of the Enlightenment is told
through his three seemingly disparate passions: medicine, politics and fossils.
As a political radical, Parkinson was interrogated over a plot to kill King
George III and was in danger of exile. But simultaneously, he was helping Edward
Jenner set up smallpox vaccination stations across London and writing the first
scientific study of fossils in English, jump-starting a national craze. He is
one of the pioneers of "the age of wonder," forgotten to history. The author
restores him to his place in history with a portrait of him and his era.
Publisher's
description : In 1817 - two hundred years years ago - James Parkinson
(1755�1824) defined this mysterious ailment so precisely that we still diagnose
Parkinson's Disease today by recognizing the symptoms he identified. The story
of this remarkable man�s contributions to the Age of the Enlightenment is told
through his three seemingly disparate passions: medicine, politics and fossils.
As a political radical, Parkinson was interrogated over a plot to kill King
George III and was in danger of exile. But simultaneously, he was helping Edward
Jenner set up smallpox vaccination stations across London and writing the first
scientific study of fossils in English, jump-starting a national craze. He is
one of the pioneers of "the age of wonder," forgotten to history. The author
restores him to his place in history with a portrait of him and his era.