.gif) VIARTIS
|
||||||
|
PARKINSON'S DISEASE |
||||||
|
|
||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
||||
|
JUNE 2012
26th June 2012 - News release LCIG CLINICAL TRIAL RESULTS FOR PARKINSON'S DISEASE Levodopa-carbidopa is administered in gel form, directly into the small intestine via a procedurally-implanted tube connected to a portable pump that delivers continuous supply of LCIG during waking hours. LCIG is currently approved in 40 countries. In the U.S.A., LCIG is an investigational therapy that is currently being evaluated in patients with advanced Parkinson's disease in additional Phase 3 clinical trials. Abbott have announced results from five abstracts evaluating levodopa-carbidopa intestinal gel (LCIG), concerning a 54 week open-label safety and tolerability study of people with advanced Parkinson's disease. The efficacy of LCIG was compared to that of standard levodopa-carbidopa immediate release, which is the same as Sinemet. There was nearly 2 hours less off time with LCIG.
For the full details go to the News release For a printable version of this article click here. In order to refer to this article on its own click here.
23rd June 2012 - New research SLEEP BENEFIT EXPERIENCED IN PARKINSON'S DISEASE
Journal of Parkinson's Disease [2012] DOI
10.3233/JPD-2012-12087 (Merrel van Gilst, Maartje Louter, Christian
Baumann, Bastiaan Bloem, Sebastiaan Overeem)
Complete study
20th June 2012 - New guidelines GUIDELINES ON PARKINSON'S DISEASE
The Guidelines are intended for a broad range of health professionals including: family physicians, neurologists, nurses, movement disorders specialists, allied health professionals (e.g. occupational therapists, physiotherapists, speech language pathologists) and other specialists. It is suggested that The Clinical Guidelines, published for the first time in 2012, will increase knowledge and will guide the diagnosis and treatment of Parkinson�s. For the guidelines go to Parkinsons Society Canada. For a printable version of this article click here. In order to refer to this article on its own click here.
16th June 2012 - New research ALCOHOL AND THE RISK OF PARKINSON'S DISEASE
Movement Disorders [2012] Jun 19 [Epub ahead of print] (Palacios N, Gao X,
O'Reilly E, Schwarzschild M, McCullough ML, Mayo T, Gapstur SM, Ascherio AA.)
Complete abstract
7th June 2012 - News release FIRST VACCINE FOR PARKINSON'S DISEASE
The vaccine is currently being tested on people with Parkinson�s Disease in a Phase I trial. The clinical trial is taking place in Vienna and involves up to 32 patients. The primary purpose is to assess the safety and tolerability of PD01A. For more information go to AFFiRis. The weakness in the theory on which the method is reliant is that a lot of people with Parkinson's Disease do not accumulate alpha-Synuclein. So there is none to get rid of. Most people that have an accumulation of alpha-Synuclein in the brain do not have Parkinson's Disease either, thereby proving that alpha-Synuclein is not the cause of Parkinson's Disease. For a printable version of this article click here. In order to refer to this article on its own click here.
4th June 2012 - New technology PARKINSON'S KINETIGRAPH The Parkinson's KinetiGraph has been devised to significantly improve the treatment available to people with Parkinson�s Disease by allowing accurate measurements of exactly how the Parkinson's Disease is affecting them.
For more information go to Parkinson's KinetiGraph. For a printable version of this article click here. In order to refer to this article on its own click here.
|
||||||
.gif) |
||||||
| �2006-2012 Viartis | ||||||
| [email protected] | ||||||
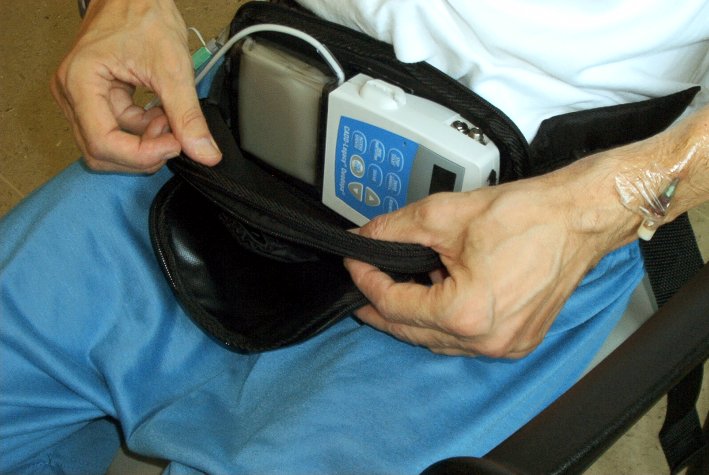 The most common adverse events were complication of device insertion (33%),
abdominal pain (30%), procedural pain (21%) and nausea (16%). The most common
serious adverse events were complication of device insertion (6%), abdominal
pain (3%) peritonitis (3%) and polyneuropathy (3%). 7% of patients withdrew due
to at least one adverse event. In another trial the most common
adverse events were complication of device insertion (51%), abdominal
pain (42%), procedural pain (32%), nausea (25%),
constipation (21%), orthostatic hypotension (18%), post-operative
wound infection (17%), and incision site erythema (16&).
The most common adverse events were complication of device insertion (33%),
abdominal pain (30%), procedural pain (21%) and nausea (16%). The most common
serious adverse events were complication of device insertion (6%), abdominal
pain (3%) peritonitis (3%) and polyneuropathy (3%). 7% of patients withdrew due
to at least one adverse event. In another trial the most common
adverse events were complication of device insertion (51%), abdominal
pain (42%), procedural pain (32%), nausea (25%),
constipation (21%), orthostatic hypotension (18%), post-operative
wound infection (17%), and incision site erythema (16&).  A
new study has confirmed that sleep improves the symptoms of nearly half (47%) of
people with Parkinson's Disease. Typically their motor functioning seems to be
better in the morning just after they have woken up. There did not seem to be a
difference in the quality of rest experienced by people who experienced the
sleep benefit and those who did not. About a third of the study participants
experienced a sleep benefit even after taking a nap. Researchers came up with
several possible reasons for the finding, though they noted that they do not
know the mechanism. When somebody lacks sleep they produce melatonin. Melatonin reduces the levels of dopamine, the substance whose deficiency causes
Parkinson's Disease. For a printable version of this article
A
new study has confirmed that sleep improves the symptoms of nearly half (47%) of
people with Parkinson's Disease. Typically their motor functioning seems to be
better in the morning just after they have woken up. There did not seem to be a
difference in the quality of rest experienced by people who experienced the
sleep benefit and those who did not. About a third of the study participants
experienced a sleep benefit even after taking a nap. Researchers came up with
several possible reasons for the finding, though they noted that they do not
know the mechanism. When somebody lacks sleep they produce melatonin. Melatonin reduces the levels of dopamine, the substance whose deficiency causes
Parkinson's Disease. For a printable version of this article
 Parkinsons
Society Canada have issued free guidelines concerning Parkinson's Disease. The
Canadian Guidelines on Parkinson�s Disease will provide health care
professionals with a detailed understanding of Parkinson�s Disease.
Parkinsons
Society Canada have issued free guidelines concerning Parkinson's Disease. The
Canadian Guidelines on Parkinson�s Disease will provide health care
professionals with a detailed understanding of Parkinson�s Disease.  Addictive
behaviours, such as cigarette smoking and coffee drinking, have been associated
with a reduced risk of Parkinson's Disease. Whether alcohol consumption is also
associated with Parkinson's Disease risk is less certain. A study has shown
that alcohol consumption was not significantly associated with the risk of
Parkinson's Disease. The likelihood of Parkinson's Disease was only slightly
elevated at 1.29 times the normal likelihood in men consuming the greatest
quantity of alcohol, but it was 0.77 times the normal
likelihood in women consuming the greatest quantity of alcohol. Overall,
consumption of beer, wine, or spirits was not associated with Parkinson's
Disease. For a printable version of this article
Addictive
behaviours, such as cigarette smoking and coffee drinking, have been associated
with a reduced risk of Parkinson's Disease. Whether alcohol consumption is also
associated with Parkinson's Disease risk is less certain. A study has shown
that alcohol consumption was not significantly associated with the risk of
Parkinson's Disease. The likelihood of Parkinson's Disease was only slightly
elevated at 1.29 times the normal likelihood in men consuming the greatest
quantity of alcohol, but it was 0.77 times the normal
likelihood in women consuming the greatest quantity of alcohol. Overall,
consumption of beer, wine, or spirits was not associated with Parkinson's
Disease. For a printable version of this article
 The
first clinical trial for the development of a Parkinson�s Disease vaccine has
been started by AFFiRiS AG. The vaccine called PD01A is directed against
alpha-Synuclein, a protein considered by AFFiRis to cause the onset and
progression of Parkinson's Disease. The vaccination aims to educate the
immune system to generate antibodies directed against alpha-Synuclein.
They believe that a reduction of the brain�s alpha-Synuclein aggregates
will have a beneficial impact on the progress of Parkinson's Disease. PD01A aims
to accomplish that by the induction of antibodies that are targeting
alpha-Synuclein, in order to neutralize its toxic impact.
The
first clinical trial for the development of a Parkinson�s Disease vaccine has
been started by AFFiRiS AG. The vaccine called PD01A is directed against
alpha-Synuclein, a protein considered by AFFiRis to cause the onset and
progression of Parkinson's Disease. The vaccination aims to educate the
immune system to generate antibodies directed against alpha-Synuclein.
They believe that a reduction of the brain�s alpha-Synuclein aggregates
will have a beneficial impact on the progress of Parkinson's Disease. PD01A aims
to accomplish that by the induction of antibodies that are targeting
alpha-Synuclein, in order to neutralize its toxic impact. 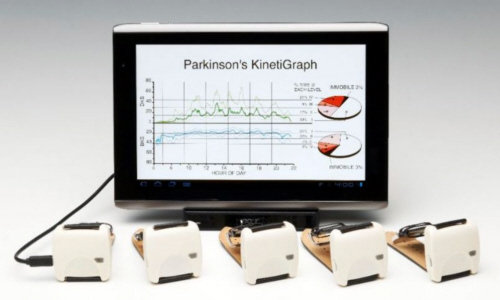 The
Parkinson�s KinetiGraph system was commercialised by Global Kinetics
Corporation. It consists of a sensor that is worn around the wrist of the
patient to record data about their symptoms, and a computer unit which receives
that data and analyses it. The device remotely records data about a person�s
movement and via algorithms, provides a report for the their neurologist showing
an objective measure of the presence and severity of bradykinesia and
dyskinesia, the two key disabling symptoms of Parkinson�s Disease. The device
also reminds the person when to take their Parkinson�s Disease drugs as
prescribed by their medical practitioner.
The
Parkinson�s KinetiGraph system was commercialised by Global Kinetics
Corporation. It consists of a sensor that is worn around the wrist of the
patient to record data about their symptoms, and a computer unit which receives
that data and analyses it. The device remotely records data about a person�s
movement and via algorithms, provides a report for the their neurologist showing
an objective measure of the presence and severity of bradykinesia and
dyskinesia, the two key disabling symptoms of Parkinson�s Disease. The device
also reminds the person when to take their Parkinson�s Disease drugs as
prescribed by their medical practitioner.