.gif) VIARTIS
|
|||||
|
PARKINSON'S DISEASE NEWS |
|||||
|
|
26th June 2012 - News release LCIG CLINICAL TRIAL RESULTS FOR PARKINSON'S DISEASE Levodopa-carbidopa is administered in gel form, directly into the small intestine via a procedurally-implanted tube connected to a portable pump that delivers continuous supply of LCIG during waking hours. LCIG is currently approved in 40 countries. In the U.S.A., LCIG is an investigational therapy that is currently being evaluated in patients with advanced Parkinson's disease in additional Phase 3 clinical trials. Abbott have announced results from five abstracts evaluating levodopa-carbidopa intestinal gel (LCIG), concerning a 54 week open-label safety and tolerability study of people with advanced Parkinson's disease. The efficacy of LCIG was compared to that of standard levodopa-carbidopa immediate release, which is the same as Sinemet. There was nearly 2 hours less off time with LCIG.
For the full details go to the News release For a printable version of this article click here. For more current news go to Parkinson's Disease News.
|
|
|||
|
Parkinson's Disease News details all significant new research, news reports, new books, and new resources concerning Parkinson's Disease and those medical disorders that often coincide with Parkinson's Disease. It is compiled from an analysis of all newly published research, news reports, new clinical trials, all newly published books, and new web sites. A summary and analysis of the new research are provided, as well as links to the complete abstracts and news reports
|
|||||
.gif) |
|||||
| �2006-2012 Viartis | |||||
| 2015-09-04 03:09:39 | |||||
| [email protected] | |||||
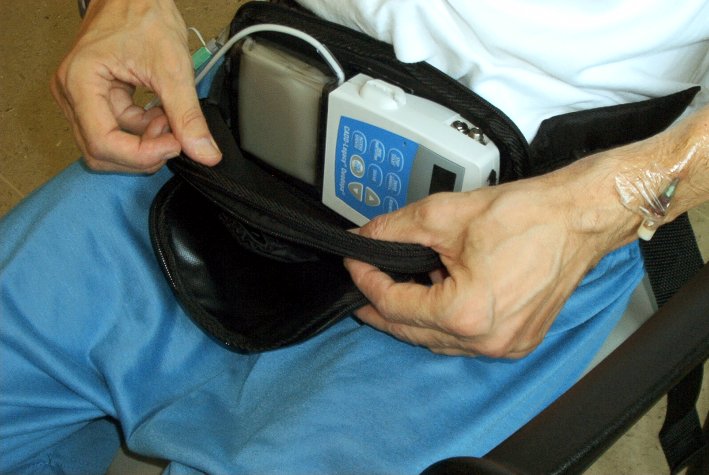 The most common adverse events were complication of device insertion (33%),
abdominal pain (30%), procedural pain (21%) and nausea (16%). The most common
serious adverse events were complication of device insertion (6%), abdominal
pain (3%) peritonitis (3%) and polyneuropathy (3%). 7% of patients withdrew due
to at least one adverse event. In another clinical trial the most common
adverse events were complication of device insertion (51%), abdominal
pain (42%), procedural pain (32%), nausea (25%),
constipation (21%), orthostatic hypotension (18%), post-operative
wound infection (17%), and incision site erythema (16%).
The most common adverse events were complication of device insertion (33%),
abdominal pain (30%), procedural pain (21%) and nausea (16%). The most common
serious adverse events were complication of device insertion (6%), abdominal
pain (3%) peritonitis (3%) and polyneuropathy (3%). 7% of patients withdrew due
to at least one adverse event. In another clinical trial the most common
adverse events were complication of device insertion (51%), abdominal
pain (42%), procedural pain (32%), nausea (25%),
constipation (21%), orthostatic hypotension (18%), post-operative
wound infection (17%), and incision site erythema (16%).  E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail
E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail