29th October 2010 - New book
PARKINSON'S DISEASE LOOKING DOWN
THE BARREL
Richard Secklin
 Publisher's
description : From contemplating suicide to a return to faith. This is a story
of my midlife encounter of getting diagnosed and living with Parkinson�s
disease. Like all PD�ers, we struggle through our own physical and/or
psychological disasters and triumphs. These are my personal experiences with
depression, confusion, the hate for God, and the on-going transition of this
diagnosis, including my symptoms, connecting with support groups, attaining
proper medical testing and treatment, and survival with
PD.
Click here for more details.
Reviewers have described it as "Eye opening and
brutally honest" and as "simply beautiful and beautifully told !" For
more books concerning Parkinson's Disease go to
Parkinson's Disease Books.
Publisher's
description : From contemplating suicide to a return to faith. This is a story
of my midlife encounter of getting diagnosed and living with Parkinson�s
disease. Like all PD�ers, we struggle through our own physical and/or
psychological disasters and triumphs. These are my personal experiences with
depression, confusion, the hate for God, and the on-going transition of this
diagnosis, including my symptoms, connecting with support groups, attaining
proper medical testing and treatment, and survival with
PD.
Click here for more details.
Reviewers have described it as "Eye opening and
brutally honest" and as "simply beautiful and beautifully told !" For
more books concerning Parkinson's Disease go to
Parkinson's Disease Books.
27th October 2010 - New research
NEURTURIN FAILS CLINICAL TRIALS FOR PARKINSON'S
DISEASE
Lancet Neurology [2010] Oct 20
[Epub ahead of print] (Marks WJ Jr, Bartus RT, Siffert J, Davis CS, Lozano A,
Boulis N, Vitek J, Stacy M, Turner D, Verhagen L, Bakay R, Watts R, Guthrie B,
Jankovic J, Simpson R, Tagliati M, Alterman R, Stern M, Baltuch G, Starr PA,
Larson PS, Ostrem JL, Nutt J, Kieburtz K, Kordower JH, Olanow CW.)
Complete abstract
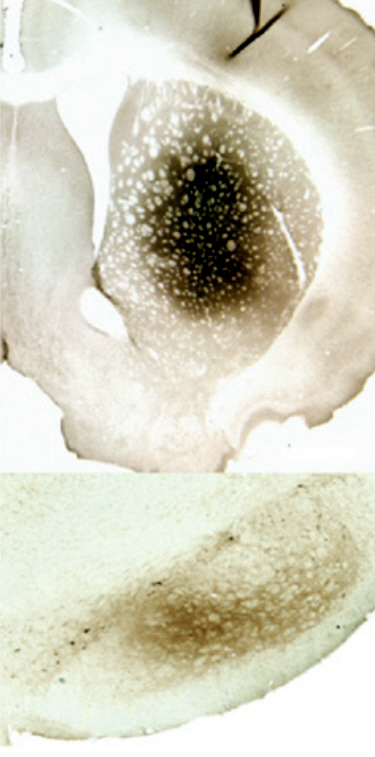 Neurturin
was hailed by its sponsors Ceregene and the Michael J Fox Foundation as having
great potential in the treatment of Parkinson's Disease, but has been found to
be completely ineffective. Neurturin (CERE-120) is an experimental drug that
contains the human gene for a growth factor called neurturin. It is used to
deliver the neurturin gene by surgical means, directly into the brain of people
with Parkinson's Disease. It was found to be worse than doing nothing, as it was
as ineffective as sham surgery (doing nothing) yet still caused severe adverse
events in a third of the patients. Neurturin is the same type of treatment
as GDNF
reference. GDNF was previously hailed as a great
breakthrough in the treatment of Parkinson's Disease. Yet all major clinical
trials of GDNF and Neurturin have failed.
In order to refer to this
article on its own
click here.
Neurturin
was hailed by its sponsors Ceregene and the Michael J Fox Foundation as having
great potential in the treatment of Parkinson's Disease, but has been found to
be completely ineffective. Neurturin (CERE-120) is an experimental drug that
contains the human gene for a growth factor called neurturin. It is used to
deliver the neurturin gene by surgical means, directly into the brain of people
with Parkinson's Disease. It was found to be worse than doing nothing, as it was
as ineffective as sham surgery (doing nothing) yet still caused severe adverse
events in a third of the patients. Neurturin is the same type of treatment
as GDNF
reference. GDNF was previously hailed as a great
breakthrough in the treatment of Parkinson's Disease. Yet all major clinical
trials of GDNF and Neurturin have failed.
In order to refer to this
article on its own
click here.
17th October 2010 - New research
THE RISK OF DEMENTIA IN PARKINSON'S DISEASE
MMW Fortschritte der Medizin [2010]
152 Supplement 1 : 1-6 (Von Reichmann H, Deuschl G, Riedel O, Spottke A, F�rstl
H, Henn F, Heuser I, Oertel W, Riederer P, Trenkwalder C, Dodel R, Wittchen HU.) Complete abstract
It is unknown, how frequently Parkinson's Disease
is complicated by dementia, depression and other neuro-psychiatric conditions. A
study on the epidemiology of Parkinson's Disease with dementia found that 28% of
people with Parkinson's Disease also had dementia. A third of people with
Parkinson's Disease (33%) had depression. A majority of people
with Parkinson's Disease (61%) had other significant psychopathological
syndromes. Just
 under 30% had no neuro-psychiatric disorders. The study
reveals for the first time comprehensively that the neuro-psychiatric problems
in people with Parkinson's Disease in all stages of Parkinson's Disease and even
early stages of Parkinson's Disease is considerable. Dementia is biochemically
unrelated to Parkinson's Disease. They coincide largely because they
both happen to be more common with age. Depression is a common symptom in
Parkinson's Disease because insufficient dopamine, which occurs in Parkinson's
Disease affects the emotions as well as the muscles.
In order to refer to this
article on its own
click here.
under 30% had no neuro-psychiatric disorders. The study
reveals for the first time comprehensively that the neuro-psychiatric problems
in people with Parkinson's Disease in all stages of Parkinson's Disease and even
early stages of Parkinson's Disease is considerable. Dementia is biochemically
unrelated to Parkinson's Disease. They coincide largely because they
both happen to be more common with age. Depression is a common symptom in
Parkinson's Disease because insufficient dopamine, which occurs in Parkinson's
Disease affects the emotions as well as the muscles.
In order to refer to this
article on its own
click here.
9th October 2010 - New research
PARCOPA FOR PARKINSON'S DISEASE
Movement Disorders [2010] Oct 5
(W.G.Ondo, L.Shinawi, S. Moore) Complete abstract
Parcopa
is an orally disintegrating combination of L-dopa and carbidopa for use in the
treatment of Parkinson's Disease. L-dopa and carbidopa is the same combination
that is in Sinemet. However, unlike Sinemet, Parcopa rapidly disintegrates on the tongue
and does not require water for dissolving or swallowing it. For more
information
 go
to
Parcopa. Because Parcopa disintegrates in the
mouth, it was suggested that there is the potential for it to shorten the time
taken from its consumption until the ridding or reduction of Parkinson's Disease
symptoms. This would make Parcopa advantageous over the use of Sinemet. When compared to Sinemet there were no significant
differences in any of the means of assessment, including changes in symptoms,
adverse events, or drug preference. It did not even make any difference to the time taken for the drug to start having
effect, which is the primary reason for its proposed use. However, according to
the authors "all trends did favor" Parcopa, suggesting a slight benefit.
In order to refer to this
article on its own
click here.
go
to
Parcopa. Because Parcopa disintegrates in the
mouth, it was suggested that there is the potential for it to shorten the time
taken from its consumption until the ridding or reduction of Parkinson's Disease
symptoms. This would make Parcopa advantageous over the use of Sinemet. When compared to Sinemet there were no significant
differences in any of the means of assessment, including changes in symptoms,
adverse events, or drug preference. It did not even make any difference to the time taken for the drug to start having
effect, which is the primary reason for its proposed use. However, according to
the authors "all trends did favor" Parcopa, suggesting a slight benefit.
In order to refer to this
article on its own
click here.
7th October 2010 - New research
PREDICTORS OF MORTALITY IN PARKINSON'S DISEASE
Neurology [2010]
75 (14) :
1270-1276 (Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G.)
Complete abstract
Researchers
have attempted to identify the risk factors of mortality in Parkinson's Disease.
In order of likelihood the percentage increase in those factors most predictive
of mortality were found to be :
dementia 89%,
male gender 63%, age 51%, psychotic symptoms 45%,
age of onset 40%,
and movement disability 18%. Dementia is not
 actually
a Parkinson's Disease symptom. However, it often eventually but not inevitably
coincides with it. There was no significant
impact of antipsychotic or Parkinson's Disease drugs on survival. The authors
suggest that early prevention of movement disability and development of
psychosis and dementia may be the most promising strategies to increasing life
expectancy in Parkinson's Disease. Given that no one factor has a really
profound effect, and as Parkinson's Disease is not a fatal disorder,
it appears that general health more commonly determines life expectancy in
Parkinson's Disease.
In order to refer to this
article on its own
click here.
actually
a Parkinson's Disease symptom. However, it often eventually but not inevitably
coincides with it. There was no significant
impact of antipsychotic or Parkinson's Disease drugs on survival. The authors
suggest that early prevention of movement disability and development of
psychosis and dementia may be the most promising strategies to increasing life
expectancy in Parkinson's Disease. Given that no one factor has a really
profound effect, and as Parkinson's Disease is not a fatal disorder,
it appears that general health more commonly determines life expectancy in
Parkinson's Disease.
In order to refer to this
article on its own
click here.
1st October 2010 - News release
BIOMARKERS FOR PARKINSON'S
DISEASE
 The
Michael J. Fox Foundation has launched the Parkinson�s Progression Markers
Initiative, the first ever large scale clinical study exclusively focused on
identifying and validating Parkinson�s Disease biomarkers. A biomarker, is a
substance used as an indicator of a biological state such as Parkinson's
Disease. The study will be carried out over five years at 18 sites in the
U.S.A. and Europe. It will track 400 people newly diagnosed with Parkinson's
Disease and 200 who do not. Recruitment is now under way at six sites, with all
sites expected to be recruiting by year-end. The study is testing the most
promising biomarker candidates through neuroimaging, the collection of blood,
urine, and spinal fluid, and clinical and behavioural tests.
For more information go to the News
release and to
the web page of the
Parkinson�s Progression Markers Initiative.
The
Michael J. Fox Foundation has launched the Parkinson�s Progression Markers
Initiative, the first ever large scale clinical study exclusively focused on
identifying and validating Parkinson�s Disease biomarkers. A biomarker, is a
substance used as an indicator of a biological state such as Parkinson's
Disease. The study will be carried out over five years at 18 sites in the
U.S.A. and Europe. It will track 400 people newly diagnosed with Parkinson's
Disease and 200 who do not. Recruitment is now under way at six sites, with all
sites expected to be recruiting by year-end. The study is testing the most
promising biomarker candidates through neuroimaging, the collection of blood,
urine, and spinal fluid, and clinical and behavioural tests.
For more information go to the News
release and to
the web page of the
Parkinson�s Progression Markers Initiative.
It is claimed that valid
measures could allow scientists to predict, objectively diagnose, and monitor
diseases as well as definitively determine which medications work and which will
not. It is already known that Parkinson's Disease is primarily due to
insufficient dopamine. The assessment of biomarkers for Parkinson's Disease has
always proven inadequate for diagnosing Parkinson's Disease because they can not
directly measure dopamine formation. Biomarkers are at best only indirect and
partial indicators of Parkinson's Disease and so could not provide information
concerning dopamine formation, which is already known anyway, and thereby lead
to effective treatments.
In order to refer to this
article on its own
click here.
.gif)
.gif)
 Publisher's
description : From contemplating suicide to a return to faith. This is a story
of my midlife encounter of getting diagnosed and living with Parkinson�s
disease. Like all PD�ers, we struggle through our own physical and/or
psychological disasters and triumphs. These are my personal experiences with
depression, confusion, the hate for God, and the on-going transition of this
diagnosis, including my symptoms, connecting with support groups, attaining
proper medical testing and treatment, and survival with
PD.
Publisher's
description : From contemplating suicide to a return to faith. This is a story
of my midlife encounter of getting diagnosed and living with Parkinson�s
disease. Like all PD�ers, we struggle through our own physical and/or
psychological disasters and triumphs. These are my personal experiences with
depression, confusion, the hate for God, and the on-going transition of this
diagnosis, including my symptoms, connecting with support groups, attaining
proper medical testing and treatment, and survival with
PD.
 Neurturin
was hailed by its sponsors Ceregene and the Michael J Fox Foundation as having
great potential in the treatment of Parkinson's Disease, but has been found to
be completely ineffective. Neurturin (CERE-120) is an experimental drug that
contains the human gene for a growth factor called neurturin. It is used to
deliver the neurturin gene by surgical means, directly into the brain of people
with Parkinson's Disease. It was found to be worse than doing nothing, as it was
as ineffective as sham surgery (doing nothing) yet still caused severe adverse
events in a third of the patients. Neurturin is the same type of treatment
as GDNF
Neurturin
was hailed by its sponsors Ceregene and the Michael J Fox Foundation as having
great potential in the treatment of Parkinson's Disease, but has been found to
be completely ineffective. Neurturin (CERE-120) is an experimental drug that
contains the human gene for a growth factor called neurturin. It is used to
deliver the neurturin gene by surgical means, directly into the brain of people
with Parkinson's Disease. It was found to be worse than doing nothing, as it was
as ineffective as sham surgery (doing nothing) yet still caused severe adverse
events in a third of the patients. Neurturin is the same type of treatment
as GDNF
 under 30% had no neuro-psychiatric disorders. The study
reveals for the first time comprehensively that the neuro-psychiatric problems
in people with Parkinson's Disease in all stages of Parkinson's Disease and even
early stages of Parkinson's Disease is considerable. Dementia is biochemically
unrelated to Parkinson's Disease. They coincide largely because they
both happen to be more common with age. Depression is a common symptom in
Parkinson's Disease because insufficient dopamine, which occurs in Parkinson's
Disease affects the emotions as well as the muscles.
In order to refer to this
article on its own
under 30% had no neuro-psychiatric disorders. The study
reveals for the first time comprehensively that the neuro-psychiatric problems
in people with Parkinson's Disease in all stages of Parkinson's Disease and even
early stages of Parkinson's Disease is considerable. Dementia is biochemically
unrelated to Parkinson's Disease. They coincide largely because they
both happen to be more common with age. Depression is a common symptom in
Parkinson's Disease because insufficient dopamine, which occurs in Parkinson's
Disease affects the emotions as well as the muscles.
In order to refer to this
article on its own
 go
to
go
to

 The
Michael J. Fox Foundation has launched the Parkinson�s Progression Markers
Initiative, the first ever large scale clinical study exclusively focused on
identifying and validating Parkinson�s Disease biomarkers. A biomarker, is a
substance used as an indicator of a biological state such as Parkinson's
Disease. The study will be carried out over five years at 18 sites in the
U.S.A. and Europe. It will track 400 people newly diagnosed with Parkinson's
Disease and 200 who do not. Recruitment is now under way at six sites, with all
sites expected to be recruiting by year-end. The study is testing the most
promising biomarker candidates through neuroimaging, the collection of blood,
urine, and spinal fluid, and clinical and behavioural tests.
For more information go to the
The
Michael J. Fox Foundation has launched the Parkinson�s Progression Markers
Initiative, the first ever large scale clinical study exclusively focused on
identifying and validating Parkinson�s Disease biomarkers. A biomarker, is a
substance used as an indicator of a biological state such as Parkinson's
Disease. The study will be carried out over five years at 18 sites in the
U.S.A. and Europe. It will track 400 people newly diagnosed with Parkinson's
Disease and 200 who do not. Recruitment is now under way at six sites, with all
sites expected to be recruiting by year-end. The study is testing the most
promising biomarker candidates through neuroimaging, the collection of blood,
urine, and spinal fluid, and clinical and behavioural tests.
For more information go to the