.gif) VIARTIS
|
||||||||||
|
PARKINSON'S DISEASE |
||||||||||
|
|
||||||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
||||||||
|
JANUARY 2015
23rd January 2015 - New research RELUCTANCE TO START PARKINSON'S DISEASE TREATMENT
The first study to assess the reluctance to start medication in Parkinson's
Disease has found a reluctance to begin medication that is primarily due to
a fear about side effects and a refusal to accept a diagnosis of Parkinson's
Disease. People with Parkinson's Disease and physicians therefore clearly have a very different perspective on the issue of reluctance to start medication. The researchers suggest that there is a need to bring physicians and patients with Parkinson's Disease closer to a shared vision of the problem reluctance to start medication. Reference : Parkinsonism Related Disorders [2014] 20 (6) : 608-612 (T.A.Mestre, T. Teodoro, W.Reginold, J.Graf, M.Kasten, J.Sale, M.Zurowski, J.Miyasaki, J.J.Ferreira, C. Marras) Complete abstract In order to refer to this article on its own click here
18th January 2015 - New research HYDROCARBONS INCREASE THE RISK OF PARKINSON'S DISEASE
Exposure to hydrocarbons has been found to significantly increase the
likelihood of developing Parkinson's Disease. This was based on by far the
largest assessment of its kind. Prior to this study there had not been a
consensus concerning hydrocarbons as a cause of Parkinson's Disease.
This systematic review supports a positive association between hydrocarbon exposure and Parkinson's Disease. In some people the likelihood of devloping Parkinson's Disease was four times normal. A more emphatic relationship may have been obtained if the degree of exposure was also considered. Reference : Parkinsonism Related Disorders [2014] Dec 26 [Epub ahead of print] (O.Palin, C.Herd, K.E.Morrison, A.C.Jagielski, K.Wheatley, G.N.Thomas, C.E.Clarke) Complete abstract In order to refer to this article on its own click here
10th January 2015 - New research DUAL RELEASE L-DOPA APPROVED FOR PARKINSON'S DISEASE
Rytary, which is produced by Impax, has been approved by the FDA (in the USA) for use in the treatment of Parkinson's Disease. Impax expects Rytary to be available for use in February 2015. Most forms of L-dopa are either immediate release, which can cause an excessive effect, or controlled release, which can be slow to start having effect. Rytary is advantageous due to including both immediate release and prolonged release L-dopa. Rytary can also be used for post-encephalitic parkinsonism, and
parkinsonism that may follow carbon monoxide intoxication or manganese
intoxication. Rytary is designed to address one of the most significant
unmet needs for patients living with Parkinson's disease, which is to reduce
the amount of time during the day when their symptoms are not adequately
controlled. In advanced Parkinson's Disease Rytary reduced the percentage of "off" time (by 37% to 23%) when compared to immediate-release carbidopa-levodopa. The most common adverse reactions with Rytary (in at least 5% of patients and more frequently than in placebo) were nausea, dizziness, headache, insomnia, abnormal dreams, dry mouth, dyskinesia, anxiety, constipation, vomiting, and orthostatic hypotension. The most common adverse reactions in advanced Parkinson's Disease were nausea and headache. For more information go to the News Release for 08 Jan 2015 News release In order to refer to this article on its own click here
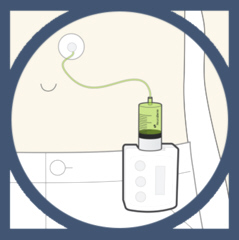
1st January 2015 - New research CLINICAL TRIALS OF SUBCUTANEOUS L-DOPA
NeuroDerm has announced results of Phase IIa pharmacokinetic Study of ND0612H and ND0612L. They led to clinically-significant plasma levels of L-dopa. ND0612 is a combination of L-dopa and carbidopa in a liquid formula administered continuously sub-cutaneously through a patch pump. ND0612 is designed to provide steady L-dopa blood levels for the reduction of motor complications in Parkinson's Disease. There is a high dose form ND0612H. For more information go to ND0612H There is a low dose form of ND0612 called ND0612L. For more information go to ND0612L
Continuous sub-cutaneous L-dopa administration using ND0612 can overcome this limitation without having to undergo invasive surgical procedure. For more information go to Neuroderm In order to refer to this article on its own click here
|
||||||||||
.gif) |
||||||||||
| �2006-2015 Viartis | ||||||||||
| [email protected] | ||||||||||
 The
most common reasons reported by patients for not starting Parkinson's
Disease treatments were : the fear of side effects (55%), followed by the
refusal to acceptance a diagnosis of Parkinson's Disease (36%). However, the
main concern of physicians in starting Parkinson's Disease drugs was their
temporary and limited benefits (34%). Patients were more than twice as
reluctant to start dopamine agonists compared with starting L-Dopa.
The
most common reasons reported by patients for not starting Parkinson's
Disease treatments were : the fear of side effects (55%), followed by the
refusal to acceptance a diagnosis of Parkinson's Disease (36%). However, the
main concern of physicians in starting Parkinson's Disease drugs was their
temporary and limited benefits (34%). Patients were more than twice as
reluctant to start dopamine agonists compared with starting L-Dopa.