.gif) VIARTIS
|
||||||
|
PARKINSON'S DISEASE |
||||||
|
|
||||||
|
|
PARKINSON'S DISEASE NEWS
|
|
||||
|
OCTOBER 2013
31st October 2013 - News release SENSORY PEN FOR DETECTING PARKINSON'S DISEASE A means of diagnosing Parkinson�s Disease is being developed by MANUS Neurodynamica using sensory pen technology. It is called thee DiPAR project. The system, combining sensor and computing technology, requires the patient to perform a set of writing tasks, drawing activities or a combination of both. The system records all movements of the pen as well as other parameters such as drawing pressure, plus acceleration and deceleration of movement, to identify patterns that are indicative of specific kinds of neuromotor disorder. The sensory pen can be used by non-specialists with minimal training so that large numbers of people would be able to be screened.
13th October 2013 - New research THE LONG TERM EFFECT OF DBS ON PARKINSON'S DISEASE Journal of the Formosan Medical Association [2013] Oct 5
[Epub ahead of print] (J.L.Jiang,
S.Y.Chen, T.C.Hsieh, C.W.Lee, S.H.Lin, S.T.Tsai)
Complete abstract
After 1 year significant improvements were seen in the UPDRS parts I, II, III, and IV and the Schwab and England scale. Five years after STN-DBS had been initiated improvements in UPDRS scores were observed only for parts II, III, and IV. In the off-medication/off-stimulation condition no significant improvement was observed. However, after 5 years there were significant deteriorations when compared to the improvements seen after 1 year in the scores for the UPDRS parts I, II, III and the Schwab and England scale.. Therefore, after the improvement experienced after 1 year the long term trend is downwards. For a printable version of this article click here. In order to refer to this article on its own click here.
7th October 2013 - New research DUAL LAYER L-DOPA CLINICAL TRIAL RESULTS Parkinsonism Related Disorders [2013] Sep 5 [Epub ahead of print]
(R.Pahwa, K.E.Lyons, R.A.Hauser, S.Fahn, J.Jankovic, E.Pourcher, A.Hsu,
M.O'Connell, S.Kell, S.Gupta)
Complete abstract
3rd October 2013 - New research DEPRESSION TREBLES THE RISK OF PARKINSON'S DISEASE Neurology [2013] Oct 2 [Epub ahead of print] (Cheng-Che Shen, Shih-Jen
Tsai, Chin-Lin Perng, Benjamin Ing-Tiau Kuo, Albert C.Yang)
Complete abstract Parkinson's Disease is primarily due to the insufficient formation of dopamine in the brain, in the dopaminergic neurons. Besides affecting muscle function and therefore the characteristic muscular symptoms of Parkinson's Disease such as as rigidity and tremor, dopamine insuffiency also affects the emotions.
3rd October 2013 - New book UNDERSTANDING PARKINSON'S DISEASE : AN INTRODUCTION FOR PATIENTS AND CAREGIVERS Naheed Ali
|
||||||
.gif) |
||||||
| �2006-2013 Viartis | ||||||
| [email protected] | ||||||
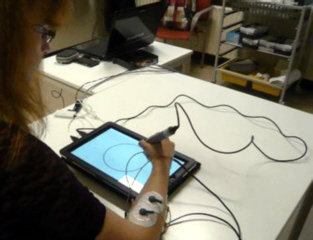 The system�s software records key features
regarding the movement of the pen, relating it to the motion of the limb,
particularly the role of the hand and fingers in coordinating overall pen
motion. The recordings enable the operator to assess
akinesia, bradykinesia, tremor, rigidity and other signs of motor
deterioration that cannot be easily detected by other means. The software takes inputs from a variety of sensors in the pen and converts them, using proprietary algorithms, into
outcome percentages that represent the likelihood of the presence of Parkinson's
Disease or
other neuromotor disorders. The method can be viewed in this brief video
The system�s software records key features
regarding the movement of the pen, relating it to the motion of the limb,
particularly the role of the hand and fingers in coordinating overall pen
motion. The recordings enable the operator to assess
akinesia, bradykinesia, tremor, rigidity and other signs of motor
deterioration that cannot be easily detected by other means. The software takes inputs from a variety of sensors in the pen and converts them, using proprietary algorithms, into
outcome percentages that represent the likelihood of the presence of Parkinson's
Disease or
other neuromotor disorders. The method can be viewed in this brief video
 The
aim of this study was to assess the improvements that can be expected after
1 year and after 5 years. Patients with Parkinson's Disease were assessed
after 1 year and 5 years according to the Unified Parkinson's disease rating
scale (UPDRS) parts I, II, III, and IV scores, the Hoehn and Yahr stage, and
Schwab and England activities of daily living (SEADL) scores in the
conditions of off-medication/on-stimulation and
off-medication/off-stimulation. Further analysis included the changes in the
L-dopa equivalent daily dose.
The
aim of this study was to assess the improvements that can be expected after
1 year and after 5 years. Patients with Parkinson's Disease were assessed
after 1 year and 5 years according to the Unified Parkinson's disease rating
scale (UPDRS) parts I, II, III, and IV scores, the Hoehn and Yahr stage, and
Schwab and England activities of daily living (SEADL) scores in the
conditions of off-medication/on-stimulation and
off-medication/off-stimulation. Further analysis included the changes in the
L-dopa equivalent daily dose. All
three dosages improved Parkinson's Disease, with the 145mg dosage, then the
245mg dosage giving better results. The most commonly reported adverse
events with IPX066 included nausea, dizziness, and headache. No unexpected
drug-related serious adverse events were reported. For a printable version of this article
All
three dosages improved Parkinson's Disease, with the 145mg dosage, then the
245mg dosage giving better results. The most commonly reported adverse
events with IPX066 included nausea, dizziness, and headache. No unexpected
drug-related serious adverse events were reported. For a printable version of this article
 This
is why dopamine insufficiency can also lead to depression. However, even
biochemically, dopamine is not the only factor involved in depression, which
is why depresssion and Parkinson's Disease do not always coincide.
Therefore, depression, even when severe, does not inevitably lead to
Parkinson's Disease and why it is possible to have Parkinson's Disease
without also having depression..
For a printable version of this article
This
is why dopamine insufficiency can also lead to depression. However, even
biochemically, dopamine is not the only factor involved in depression, which
is why depresssion and Parkinson's Disease do not always coincide.
Therefore, depression, even when severe, does not inevitably lead to
Parkinson's Disease and why it is possible to have Parkinson's Disease
without also having depression..
For a printable version of this article
 Publisher's
description : Understanding Parkinson�s Disease offers patients and their
caregivers the kind of cutting-edge information that will allow them to
successfully confront this debilitating disease on a number of fronts. Patients
will also be uniquely exposed to alternative approaches to managing the symptoms
of the disease, including allopathic, osteopathic, and naturopathic approaches.
The reader will be introduced to essential information on the risk factors
associated with Parkinson�s, the signs and symptoms, the different stages of the
disease, the various treatments, as well as how the disease develops. Anyone
looking for an introduction will find the information they need in this
accessible resource.
Publisher's
description : Understanding Parkinson�s Disease offers patients and their
caregivers the kind of cutting-edge information that will allow them to
successfully confront this debilitating disease on a number of fronts. Patients
will also be uniquely exposed to alternative approaches to managing the symptoms
of the disease, including allopathic, osteopathic, and naturopathic approaches.
The reader will be introduced to essential information on the risk factors
associated with Parkinson�s, the signs and symptoms, the different stages of the
disease, the various treatments, as well as how the disease develops. Anyone
looking for an introduction will find the information they need in this
accessible resource.