PSYCHIATRIC EFFECTS OF DBS SURGERY IN PARKINSON'S
DISEASE
Movement Disorders [2007] Aug 22; [Epub ahead of
print] (Appleby BS, Duggan PS, Regenberg A, Rabins PV.)
Complete abstract
Deep brain stimulation (DBS) has been approved by
the FDA for use in the treatment of Parkinson's disease, essential tremor, and
dystonia. Case reports and case series have reported significant psychiatric
side effects in some individuals. The goal of this study was to assess the
benefits and psychiatric risks of DBS. A search was conducted
on
PubMed, EBSCO, and PsycInfo that covered the time period 1 Jan 1996-30 Dec 2005.
 98.2% of studies that assessed motor function reported some level of
improvement. Most reported side effects were device or procedure related (e.g.,
infection and lead fracture). The prevalence of depression was 2-4%, mania
0.9-1.7%, emotional changes 0.1-0.2%, and the prevalence of suicidal
ideation/suicide attempt was 0.3-0.7%. The completed suicide rate was
0.16-0.32%. In conclusion, DBS has effect in Parkinson's disease, dystonia, and
essential tremor, but there is a high rate of suicide in patients treated with
DBS.
98.2% of studies that assessed motor function reported some level of
improvement. Most reported side effects were device or procedure related (e.g.,
infection and lead fracture). The prevalence of depression was 2-4%, mania
0.9-1.7%, emotional changes 0.1-0.2%, and the prevalence of suicidal
ideation/suicide attempt was 0.3-0.7%. The completed suicide rate was
0.16-0.32%. In conclusion, DBS has effect in Parkinson's disease, dystonia, and
essential tremor, but there is a high rate of suicide in patients treated with
DBS.
29th August 2007 : News report
PARKINSON'S GOGGLES
About
40% of people with Parkinson's disease suffer from gait freeze, walking
difficulties, difficulty turning corners, small shuffling steps. They may also
find it hard to cross thresholds, and so freeze if the floor surface or colour
changes. Parkinson's goggles are designed to help patients feel like they are
walking on a steady floor.
 The goggles have a sensor that activates an LCD
screen to show patients a tile pattern on the floor. The checkerboard pattern
projected on the floor makes them feel like they have something to step over.
There is also an auditory feedback cue. The goggles are still being tested.
About 10 patients have tried them so far in this study, and the goal is 20
people. Patients in the study wore the goggles for a half hour a day for two
weeks, and some seem to still see the tiles after they take off the goggles. For
more information go to the Complete
article.
The goggles have a sensor that activates an LCD
screen to show patients a tile pattern on the floor. The checkerboard pattern
projected on the floor makes them feel like they have something to step over.
There is also an auditory feedback cue. The goggles are still being tested.
About 10 patients have tried them so far in this study, and the goal is 20
people. Patients in the study wore the goggles for a half hour a day for two
weeks, and some seem to still see the tiles after they take off the goggles. For
more information go to the Complete
article.
28th August 2007 : News report
Transdermal
Rotigotine as Safe and Effective as Pramipexole
A transdermal patch system with rotigotine, a
dopamine agonist, appears safe and shows efficacy comparable with pramipexole
and better than placebo for people with advanced Parkinson's disease say
researchers. The abdominal patch application, recently licensed in Europe for
use in early and late Parkinson's Disease, has been shown to provide maintained
mean plasma rotigotine concentrations. The
primary efficacy measure was mean decrease in hours per day in "off" time,
with equivalent significant improvements over placebo (0.88) maintained for
pramipexole (-2.8) and rotigotine (-2.5).
The time spent "on without troublesome dyskinesis" (hours/day) was increased
over placebo (1.5) for both pramipexole (3.0) and rotigotine (3.1). The
frequencies of adverse events were
similar across the three treatments.
 Those most common for rotigotine were
nausea (17%), dyskinesia (12%), somnolence (12%), and application-site erythema/pruritus
(9%). The work was carried out for Schwarz Pharma, who are developing
transdermal rotigotine. For more information go to the Complete
article. This study was published in
Lancet Neurology [2007] 6 : 513-530 (Poewe WH, Rascol O, Quinn N et al)
Complete
abstract. Unfortunately,
the problem with all dopamine agonists is that they become progressively
counterproductive because they decrease the sensitivity of the
dopamine receptors that they initially stimulate.
Those most common for rotigotine were
nausea (17%), dyskinesia (12%), somnolence (12%), and application-site erythema/pruritus
(9%). The work was carried out for Schwarz Pharma, who are developing
transdermal rotigotine. For more information go to the Complete
article. This study was published in
Lancet Neurology [2007] 6 : 513-530 (Poewe WH, Rascol O, Quinn N et al)
Complete
abstract. Unfortunately,
the problem with all dopamine agonists is that they become progressively
counterproductive because they decrease the sensitivity of the
dopamine receptors that they initially stimulate.
24th August 2007 : New research
L-DOPA METABOLITE WORSENS PARKINSON'S DISEASE
Neurochemical research [2007] August 24th [Epub
ahead of print] (Lee ES, Chen H, King J, Charlton C.)
Complete
abstract
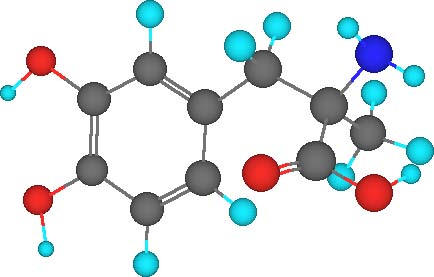
The cause of the L-dopa's adverse effects has not
been established to date. 3-O-methyldopa (3-OMD), which is a major metabolite of
L-dopa, was tested to determine whether it plays a role in the adverse effects.
3-OMD impaired
locomotor
activities by decreasing movement time, total distance, and number of movement
by 70%, 74% and 61%, respectively.
 It decreased the dopamine turnover rate by
40%, and inhibited dopamine transporter and uptake. 3-OMD induced cytotoxic
effects via oxidative stress and decreased mitochondrial membrane potential,
indicating that it can also damage neuronal cells. 3-OMD also potentiated L-dopa
toxicity. Therefore, L-dopa treatment can accelerate the progression of
Parkinson's Disease because of its metabolite 3-O-Methyldopa.
It decreased the dopamine turnover rate by
40%, and inhibited dopamine transporter and uptake. 3-OMD induced cytotoxic
effects via oxidative stress and decreased mitochondrial membrane potential,
indicating that it can also damage neuronal cells. 3-OMD also potentiated L-dopa
toxicity. Therefore, L-dopa treatment can accelerate the progression of
Parkinson's Disease because of its metabolite 3-O-Methyldopa.
23rd August 2007 : New research
COFFEE AND TEA LOWER THE RISK OF PARKINSON'S
DISEASE
Movement Disorders [2007] August 21st [ahead of print]
(Hu
G,
Bidel S,
Jousilahti P,
Antikainen R,
Tuomilehto J.)
Complete
abstract
 Several
prospective studies have assessed the association between coffee consumption and
Parkinson's disease risk, but the results were inconsistent. A massive study was
carried out in Finland on nearly 30,000 subjects. They examined the association
of coffee and tea consumption with the risk of Parkinson's Disease. There
was a clear trend in both men and women for coffee drinkers to be less prone to
Parkinson's Disease. In both sexes combined, the multivariate-adjusted
likelihood for subjects drinking 3 or more cups of tea per day compared to
non-tea drinkers also showed a clearly lower risk of Parkinson's Disease.
Several
prospective studies have assessed the association between coffee consumption and
Parkinson's disease risk, but the results were inconsistent. A massive study was
carried out in Finland on nearly 30,000 subjects. They examined the association
of coffee and tea consumption with the risk of Parkinson's Disease. There
was a clear trend in both men and women for coffee drinkers to be less prone to
Parkinson's Disease. In both sexes combined, the multivariate-adjusted
likelihood for subjects drinking 3 or more cups of tea per day compared to
non-tea drinkers also showed a clearly lower risk of Parkinson's Disease.
23rd August 2007 : New clinical trial
NEW CLINICAL TRIAL OF THE JACOBSON RESONATOR
This study will evaluate a new non-invasive, non
significant risk device therapy as a supplemental symptomatic treatment for
Parkinson�s Disease. For more information go to the Complete
article. The device utilizes technology involving extremely low level
electromagnetic fields (EMF) to provide whole body immersion. EMF treatment with
the Jacobson Resonator is a new technology. For more information go to
Magnetic therapy. It has shown promising results in a Phase I pilot study
earlier in 2007, which preceded this Phase II study.
23rd August 2007 : News report
FDA CONSIDER FIRST ADULT STEM CELL TRIALS FOR
PARKINSON'S DISEASE
 Biopharmaceutical company
BrainStorm Cell
Therapeutics has arranged a meeting
with the U.S. Food and Drug Administration (FDA) to coordinate clinical trials
for its flagship product for the treatment of Parkinson's disease. The company
said it aims to be the first worldwide to win approval to conduct trials using
adult stem cells taken from patients. BrainStorm has hired Dr Andra E.Miller,
director of cell and gene therapies of the Biologics Consulting Group Inc. to
handle the FDA negotiations.
For more information go to the Complete
article.
Biopharmaceutical company
BrainStorm Cell
Therapeutics has arranged a meeting
with the U.S. Food and Drug Administration (FDA) to coordinate clinical trials
for its flagship product for the treatment of Parkinson's disease. The company
said it aims to be the first worldwide to win approval to conduct trials using
adult stem cells taken from patients. BrainStorm has hired Dr Andra E.Miller,
director of cell and gene therapies of the Biologics Consulting Group Inc. to
handle the FDA negotiations.
For more information go to the Complete
article.
22nd August 2007 : News report
SAFINAMIDE FAILS IN CLINICAL TRIALS
Merck and Newron plan further tests on their
safinamide drug for Parkinson's disease with a lower dose after a late trial
showed a higher dose lacked any significant effect. The 18-month clinical trial
pooled data from patients taking both the 50-100 mg and 150-200 mg doses who had
had the disease for less than five years. "The primary endpoint, time to
intervention, did not reach statistical significance when data from both
safinamide dose groups were pooled," the companies said in a statement.
 They
said their analysis showed that the lower doses of safinamide
alongside dopamine led to improvements and reduced the need to make changes to
treatment regimes. "Additional Phase III studies are planned to further assess
the efficacy of this dose of safinamide," they added. For more information
go to the Complete
article. Safinamide has a novel dual mechanism of action based on the
enhancement of the dopaminergic function (through potent, reversible inhibition
of MAO-B, and dopamine uptake) and reduction of glutamatergic activity by
inhibiting glutamate release. For more information go to
Safinamide.
They
said their analysis showed that the lower doses of safinamide
alongside dopamine led to improvements and reduced the need to make changes to
treatment regimes. "Additional Phase III studies are planned to further assess
the efficacy of this dose of safinamide," they added. For more information
go to the Complete
article. Safinamide has a novel dual mechanism of action based on the
enhancement of the dopaminergic function (through potent, reversible inhibition
of MAO-B, and dopamine uptake) and reduction of glutamatergic activity by
inhibiting glutamate release. For more information go to
Safinamide.
19th August 2007 : New research
GDNF FAILS TO HAVE ANY BENEFIT IN PARKINSON'S
DISEASE
Acta
Neurochirurgica Supplement [2007] 97 (Part 2) : 135-154 (N.K.Patel,
S.S.Gill)
Complete abstract
Amgen previously carried out a trial using GDNF. Patients claimed it to be a
cure. Despite ongoing protests, Amgen ceased its use because they claimed it was
toxic and ineffective. For more information go to Complete
article. GDNF is a neurotrophic factor - a protein that regulates
neuronal survival, differentiation, growth and regeneration. It is claimed
to represent an alternative for treating dopaminergic neurons in Parkinson's
Disease but is difficult to administer clinically because it does not pass
through the blood-brain
barrier.
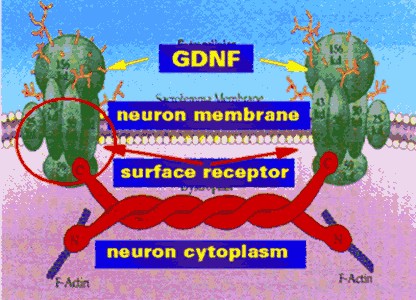 In an independent trial in England, continuous GDNF infusion in five patients
showed good tolerance, few side effects and clinical benefit within 3 months of
treatment. The clinical improvement was sustained and progressive. A
second clinical trial in ten patients also showed a greater than 30% bilateral
benefit in both on and off-medication score at 24 weeks. A randomized controlled
clinical trial was recently conducted to confirm the previous clinical trial
results. However, GDNF infusion over 6 months did not confer the predetermined
level of clinical benefit. The researchers suggest that technical differences
between the recent trial and previous studies contributed to this negative
outcome.
In an independent trial in England, continuous GDNF infusion in five patients
showed good tolerance, few side effects and clinical benefit within 3 months of
treatment. The clinical improvement was sustained and progressive. A
second clinical trial in ten patients also showed a greater than 30% bilateral
benefit in both on and off-medication score at 24 weeks. A randomized controlled
clinical trial was recently conducted to confirm the previous clinical trial
results. However, GDNF infusion over 6 months did not confer the predetermined
level of clinical benefit. The researchers suggest that technical differences
between the recent trial and previous studies contributed to this negative
outcome.
15th August 2007 : News report
TWO PARKINSON'S DISEASE DRUGS CAUSE HEART RISKS
Two drugs used to treat Parkinson's Disease have
been found to cause damage to heart valves. The drugs, pergolide (Permax) and
cabergoline (Dostinex or Cabaser), are dopamine agonists. Researchers
looked at the medical records of 11,417 patients who had
received Parkinson's drugs between 1988 and 2005.
 They found that pergolide and
cabergolide increase the risk of a heart valve condition by 7 and 5 times, when
compared to another Parkinson's Disease drug. Patients on Parkinson's drugs
besides these did not increase their risk of developing the condition.
Permax had already been withdrawn from sale in the U.S.A..
For full details go to the
Complete
article. Canadian Health Authorities have now ordered the cessation of the
sale of Permax in Canada.
For full details go to the
Complete
article.
They found that pergolide and
cabergolide increase the risk of a heart valve condition by 7 and 5 times, when
compared to another Parkinson's Disease drug. Patients on Parkinson's drugs
besides these did not increase their risk of developing the condition.
Permax had already been withdrawn from sale in the U.S.A..
For full details go to the
Complete
article. Canadian Health Authorities have now ordered the cessation of the
sale of Permax in Canada.
For full details go to the
Complete
article.
14th August 2007 : New resource
PARKINSON'S DISEASE BLOG NETWORK

The Parkinson's Disease Blog Network is a new
Parkinson's Disease resource set up to centralize Parkinson's Disease blogs. It
was developed and is maintained by Incendia Health Studios, an inVentiv Health
company. It has been designed to serve as a real-time, virtual Parkinson's
disease discussion and community forum, allowing users to exchange information
and thoughts on topics ranging from symptoms to diet and treatment. All blogs
registered on the network are listed with a brief descriptor and assigned to one
of three categories : resource, medical or personal. Each includes spaces for
user comments, a five-point rating scale, and tracks the number of "views". This
enables the blogs found to be the most useful to be the most prominently
featured. For their web site go to the
Parkinson's Disease Blog Network
14th August 2007 : News report
OVER 60% OF NEWLY DIAGNOSED RECEIVE NO DRUG
TREATMENT IN THE FIRST YEAR
Decision Resources, one of the world's leading
research and advisory firms found that 61 percent of newly diagnosed Parkinson's
disease patients do not receive any drug treatment in the first year of
diagnosis. According to the new report it is likely that this occurs as a result
of patients' decisions to delay treatment until symptoms become sufficiently
troublesome. Furthermore, patients may be reluctant to begin L-dopa therapy,
knowing that side-effects will arise.
For full details go to the
Complete
article.
13th August 2007 : New
books
PARKINSON'S DISEASE and related
disorders (PARTS 1 AND 2)
William C.Koller, Eldad Melamed

 The
two volumes on Parkinsons disease in the Handbook of Clinical Neurology
cover the dramatic advances in understanding of the biochemical background of
Parkinsonism and the resulting developments in its pharmacological management.
These volumes give an account of the subject for both clinical neurologists and
those researching in the neurosciences. Part I covers the scientific background,
general aspects of Parkinson's disease, clinical aspects and etiology. Part II
covers the medical and surgical treatment of Parkinsons disease, complications
of therapy and the other parkinsonian syndromes.
For details of Part I go to
Book details. For details of Part II go to
Book details.
The
two volumes on Parkinsons disease in the Handbook of Clinical Neurology
cover the dramatic advances in understanding of the biochemical background of
Parkinsonism and the resulting developments in its pharmacological management.
These volumes give an account of the subject for both clinical neurologists and
those researching in the neurosciences. Part I covers the scientific background,
general aspects of Parkinson's disease, clinical aspects and etiology. Part II
covers the medical and surgical treatment of Parkinsons disease, complications
of therapy and the other parkinsonian syndromes.
For details of Part I go to
Book details. For details of Part II go to
Book details.
13th August 2007 : New research
IS PARKINSON'S DISEASE DUE TO A LOSS OF TWO CELL
TYPES RATHER THAN ONE ?
Authors of new research are
claiming that the loss of two types of brain cells - not just one as previously
thought - may trigger Parkinson's disease. The evidence, based on mouse models,
shows a link between the loss of both norepinephrine and dopamine neurons and
the delayed onset of symptoms associated with Parkinson's disease.
 They suggest
that it's possible that simultaneously treating both the dopamine and
norepinephrine loss could further ameliorate Parkinson's disease.
For full details go to the
Complete
article. The results will be published in the Proceedings of the National
Academy of Sciences, Early Edition online during the week of August 13th-17th.
Although the authors claim that there is loss of dopamine producing cells in
Parkinson's Disease, no research has ever shown this. Dopamine producing cells
have only ever been shown to reduce their activity in Parkinson's Disease. Also,
norepinephrine is made via the same biochemical means as dopamine, so it is
obvious that a lack of dopamine will be associated with a lack of norepinephrine.
They suggest
that it's possible that simultaneously treating both the dopamine and
norepinephrine loss could further ameliorate Parkinson's disease.
For full details go to the
Complete
article. The results will be published in the Proceedings of the National
Academy of Sciences, Early Edition online during the week of August 13th-17th.
Although the authors claim that there is loss of dopamine producing cells in
Parkinson's Disease, no research has ever shown this. Dopamine producing cells
have only ever been shown to reduce their activity in Parkinson's Disease. Also,
norepinephrine is made via the same biochemical means as dopamine, so it is
obvious that a lack of dopamine will be associated with a lack of norepinephrine.
12th August 2007 : New research
DOES URIC ACID LOWER THE CHANCE OF GETTING
PARKINSON'S DISEASE ?
American Journal of Epidemiology
[2007] - Advance Access published online (MG Weisskopf,
E O'Reilly, H Chen, MA Schwarzschild and
A Ascherio)
In 1996 scientists
reported that men with above average serum uric acid levels had a 40% reduction
in their likelihood of later developing Parkinson's disease
Abstract. The scientists suggested that the antioxidant properties of uric
acid might protect against oxidative damage and nerve cell death in Parkinson's
Disease. A recent follow up study, using a larger population showed that
participants with the highest uric acid levels had a 55% lower likelihood of
developing Parkinson's Disease than those participants with the lowest uric acid
levels
Abstract. The principal investigator consequently claimed that uric acid
could become the first biomarker of Parkinson's disease.
For
full details go to the
Complete
article.
 However, there is only a general tendency for Parkinson's Disease
to be associated with uric acid levels. Uric acid levels could therefore not
determine whether or not somebody had or would have Parkinson's Disease. Also,
the reason for this association is likely to be different from the explanation
given. High protein diets, likely to produce uric acid, are also high in the
L-dopa precursors L-tyrosine and L-phenylalanine. L-tyrosine and
L-phenylalanine would lessen the tendency towards Parkisnon's Disease because
they can increase dopamine levels. For more information on
Uric acid.
However, there is only a general tendency for Parkinson's Disease
to be associated with uric acid levels. Uric acid levels could therefore not
determine whether or not somebody had or would have Parkinson's Disease. Also,
the reason for this association is likely to be different from the explanation
given. High protein diets, likely to produce uric acid, are also high in the
L-dopa precursors L-tyrosine and L-phenylalanine. L-tyrosine and
L-phenylalanine would lessen the tendency towards Parkisnon's Disease because
they can increase dopamine levels. For more information on
Uric acid.
11th August 2007 : News report
Michael J.
Fox Foundation Announces $1.2 Million in Funding
 The Michael J. Fox Foundation for
Parkinson's Research announced $1.2 million in awards to four research teams working
in the drug development pipeline. The research projects will be :
the autophagy pathway, a cellular pathway involved
in clearing away protein such as alpha-synuclein; optimizing administration of
the compound MX-4565, a non-feminizing estrogen analog that has been shown to be
effective in protecting nerve cells from toxic stresses; the potential of the
protein osteopontin as a Parkinson's Disease treatment; and a molecule called
fisetin, which can maintain levels of glutathione, which is produced naturally
as a defence against oxidative stress.
The Michael J. Fox Foundation for
Parkinson's Research announced $1.2 million in awards to four research teams working
in the drug development pipeline. The research projects will be :
the autophagy pathway, a cellular pathway involved
in clearing away protein such as alpha-synuclein; optimizing administration of
the compound MX-4565, a non-feminizing estrogen analog that has been shown to be
effective in protecting nerve cells from toxic stresses; the potential of the
protein osteopontin as a Parkinson's Disease treatment; and a molecule called
fisetin, which can maintain levels of glutathione, which is produced naturally
as a defence against oxidative stress.
9th August 2007 : New clinical trial
CREATINE TO BE TESTED AS A TREATMENT FOR
PARKINSON'S DISEASE
Creatine is an acid naturally produced by the body
for regulating cell energy. For more information go to
Creatine. Because some evidence suggests it may
have antioxidant properties, creatine is also marketed as a nutritional
supplement. Avicena Group, Inc. a biotechnology company are supplying the
creatine and a placebo for a NINDS study involving 1,720 patients.
 The drug
being tested is significantly different from over-the-counter creatine
supplements. Efficacy will be determined over 5 to 7 years by means of tests
that measure ability to walk, carry out other daily activities, cognitive
function, and general quality of life. For full details go to the
Complete article. There are weaknesses in this study. Even in theory
creatine does nothing to raise dopamine levels, which is the fundamental aim in
Parkinson's Disease. There is no need to take creatine because the body can make
its own from common dietary substances. In previous studies, creatine was not
really found to be effective in Parkinson's Disease.
The drug
being tested is significantly different from over-the-counter creatine
supplements. Efficacy will be determined over 5 to 7 years by means of tests
that measure ability to walk, carry out other daily activities, cognitive
function, and general quality of life. For full details go to the
Complete article. There are weaknesses in this study. Even in theory
creatine does nothing to raise dopamine levels, which is the fundamental aim in
Parkinson's Disease. There is no need to take creatine because the body can make
its own from common dietary substances. In previous studies, creatine was not
really found to be effective in Parkinson's Disease.
8th August 2007 : News report
FDA APPROVE HIGHER DOSE OF STALEVO FOR PARKINSON'S
DISEASE
 The U.S. Food and Drug Administration (FDA) has
approved a new higher dose strength of Stalevo (carbidopa, levodopa and
entacapone). Stalevo is indicated for Parkinson's disease patients with signs
and symptoms of end-of-dose "wearing off." The approval of the higher 200mg
Stalevo (50 mg carbidopa, 200 mg levodopa, 200 mg entacapone) gives physicians
greater dosing flexibility in the treatment of patients experiencing symptom
re-emergence due to end of dose wearing off. The new dosage strength may lessen
the burden of managing multiple medications.
For full details go to the
Complete article. For more
information on Stalevo.
The U.S. Food and Drug Administration (FDA) has
approved a new higher dose strength of Stalevo (carbidopa, levodopa and
entacapone). Stalevo is indicated for Parkinson's disease patients with signs
and symptoms of end-of-dose "wearing off." The approval of the higher 200mg
Stalevo (50 mg carbidopa, 200 mg levodopa, 200 mg entacapone) gives physicians
greater dosing flexibility in the treatment of patients experiencing symptom
re-emergence due to end of dose wearing off. The new dosage strength may lessen
the burden of managing multiple medications.
For full details go to the
Complete article. For more
information on Stalevo.
7th August 2007 : New research
PREDICTORS OF FREEZING IN PARKINSON'S DISEASE
Movement Disorders [2007] 22 (7) : 953-956
(Macht
M,
Kaussner Y,
Moller JC,
Stiasny-Kolster K,
Eggert KM,
Kruger HP,
Ellgring H.)
Complete abstract
 A
survey of over 6000 Parkinson's disease patients were examined for the
relationship between freezing and age, gender, duration, subjective severity of
Parkinson's disease, and antiparkinsonian medication. 47% of people with
Parkinson's Disease reported experiencing freezing regularly. Freezing was
associated with longer disease duration and the more advanced stages of
Parkinson's Disease. Freezing episodes were more likely in men than in women.
Freezing was more common in patients taking, in addition to L-dopa, Entacapone,
Amantadine, or dopamine agonists. Patients considering tremor as their main
symptom reported freezing less frequently. Common Parkinson's Disease drugs
given in combination with L-dopa were not negatively related to episodes of
freezing.
A
survey of over 6000 Parkinson's disease patients were examined for the
relationship between freezing and age, gender, duration, subjective severity of
Parkinson's disease, and antiparkinsonian medication. 47% of people with
Parkinson's Disease reported experiencing freezing regularly. Freezing was
associated with longer disease duration and the more advanced stages of
Parkinson's Disease. Freezing episodes were more likely in men than in women.
Freezing was more common in patients taking, in addition to L-dopa, Entacapone,
Amantadine, or dopamine agonists. Patients considering tremor as their main
symptom reported freezing less frequently. Common Parkinson's Disease drugs
given in combination with L-dopa were not negatively related to episodes of
freezing.
4th August 2007 : New
research
Electromagnetic stimulation reduces Parkinson's Disease
Movement Disorders [2007] 22 (7)
: 1046-1050 (Khedr EM, Rothwell JC, Shawky OA, Ahmed MA, Foly N, Hamdy A.)
Complete abstract
Transcranial magnetic stimulation
reduced Parkinson's Disease.
Repeated sessions of repetitive transcranial magnetic stimulation
(rTMS) have been reported to produce significant improvement of motor
performance in patients with Parkinson's Disease. This study assessed whether
repeated sessions of rTMS increase serum dopamine in PD patients and whether
this correlates with changes in clinical rating scales.
 There was significant
improvement in UPDRS (the major method of Parkinson's Disease assessment)
compared with the baseline. Serum dopamine level also was significantly elevated
over the same interval. There was a significant correlation between UPDRS and
serum dopamine level before and after treatment. Therefore, improved motor
performance in PD after repeated session of rTMS may be related to an elevation
of serum dopamine concentration.
What needs to be assessed is the effects of long term use, and
any negative effects.
For more information on
Transcranial magnetic stimulation
There was significant
improvement in UPDRS (the major method of Parkinson's Disease assessment)
compared with the baseline. Serum dopamine level also was significantly elevated
over the same interval. There was a significant correlation between UPDRS and
serum dopamine level before and after treatment. Therefore, improved motor
performance in PD after repeated session of rTMS may be related to an elevation
of serum dopamine concentration.
What needs to be assessed is the effects of long term use, and
any negative effects.
For more information on
Transcranial magnetic stimulation
2nd August 2007 : New
research
How Alpha-Synuclein can cause Parkinson's Disease
Neuroscience Bulletin [2007] 23
(1) : 53-57 (Gao N, Li YH, Li X, Yu S, Fu GL, Chen B.)
Complete
abstract
 Alpha synuclein has been known to
increase Parkinson's Disease, but the means has not been known. New research has
shown that alpha-synuclein genetically interferes with the formation of tyrosine
hydroxylase. Tyrosine hydroxylase is the enzyme needed to form dopamine via the
following means L-tyrosine > L-dopa > dopamine. Dopamine formation is essential
for the relief of Parkinson's Disease. So Alpha Synuclein causes Parkinson's
Disease symptoms by reducing dopamine formation. For more information on
Alpha-Synuclein
Alpha synuclein has been known to
increase Parkinson's Disease, but the means has not been known. New research has
shown that alpha-synuclein genetically interferes with the formation of tyrosine
hydroxylase. Tyrosine hydroxylase is the enzyme needed to form dopamine via the
following means L-tyrosine > L-dopa > dopamine. Dopamine formation is essential
for the relief of Parkinson's Disease. So Alpha Synuclein causes Parkinson's
Disease symptoms by reducing dopamine formation. For more information on
Alpha-Synuclein
1st August 2007 : New
research
Is L-dopa also good for Restless Legs Syndrome ?
Movement Disorders [2007] Jul 20;
[Epub ahead of print] (Conti CF, de Oliveira MM, Andriolo RB, Saconato H,
Atallah AN, Valbuza JS, Coin de Carvalho LB, do Prado GF.)
Complete
abstract
 The treatment of Restless Legs
Syndrome using L-dopa has been claimed. An assessment was made of all the
clinical trials that had used L-dopa for Restless Legs Syndrome.
Nine eligible clinical trials
were included. The subjective analyses of these studies showed contradictory
results, but the objective analyses showed that treatment group had a
statistically significant improvement of periodic leg movement (PLM) index,
favouring the treatment group. The most common adverse event seen was
gastrointestinal symptoms. The short-term treatment with L-dopa was effective
and safe for PLM, but there were only a few trials assessing long-term treatment
in RLS. As
the
assessment gave inconclusive results, it suggests that Restless Legs Syndrome is
not simply due to a lack of L-dopa and dopamine as has been theorised. For more
information on
Restless Legs Syndrome
The treatment of Restless Legs
Syndrome using L-dopa has been claimed. An assessment was made of all the
clinical trials that had used L-dopa for Restless Legs Syndrome.
Nine eligible clinical trials
were included. The subjective analyses of these studies showed contradictory
results, but the objective analyses showed that treatment group had a
statistically significant improvement of periodic leg movement (PLM) index,
favouring the treatment group. The most common adverse event seen was
gastrointestinal symptoms. The short-term treatment with L-dopa was effective
and safe for PLM, but there were only a few trials assessing long-term treatment
in RLS. As
the
assessment gave inconclusive results, it suggests that Restless Legs Syndrome is
not simply due to a lack of L-dopa and dopamine as has been theorised. For more
information on
Restless Legs Syndrome
ARCHIVES :
.gif)
.gif)
 Recent studies report that exposure to manganese, an essential component of
welding and some steels, results in
neurotoxicity or Parkinson's Disease in welders. This review presents a critical
analysis of the published studies that were
conducted on a variety of Manganese exposed occupational cohorts
during the last hundred years, and the regulatory
history of Manganese and welding fumes. Data indicates that Manganese exposures
in welders are less than those associated with the reports of manganism in
miners and smelter workers. It was also found that although manganism was
observed in very highly exposed workers, that the scant exposure data available
for welders do not support a conclusion that welding is associated with
neurotoxicity.
Recent studies report that exposure to manganese, an essential component of
welding and some steels, results in
neurotoxicity or Parkinson's Disease in welders. This review presents a critical
analysis of the published studies that were
conducted on a variety of Manganese exposed occupational cohorts
during the last hundred years, and the regulatory
history of Manganese and welding fumes. Data indicates that Manganese exposures
in welders are less than those associated with the reports of manganism in
miners and smelter workers. It was also found that although manganism was
observed in very highly exposed workers, that the scant exposure data available
for welders do not support a conclusion that welding is associated with
neurotoxicity. 98.2% of studies that assessed motor function reported some level of
improvement. Most reported side effects were device or procedure related (e.g.,
infection and lead fracture). The prevalence of depression was 2-4%, mania
0.9-1.7%, emotional changes 0.1-0.2%, and the prevalence of suicidal
ideation/suicide attempt was 0.3-0.7%. The completed suicide rate was
0.16-0.32%. In conclusion, DBS has effect in Parkinson's disease, dystonia, and
essential tremor, but there is a high rate of suicide in patients treated with
DBS.
98.2% of studies that assessed motor function reported some level of
improvement. Most reported side effects were device or procedure related (e.g.,
infection and lead fracture). The prevalence of depression was 2-4%, mania
0.9-1.7%, emotional changes 0.1-0.2%, and the prevalence of suicidal
ideation/suicide attempt was 0.3-0.7%. The completed suicide rate was
0.16-0.32%. In conclusion, DBS has effect in Parkinson's disease, dystonia, and
essential tremor, but there is a high rate of suicide in patients treated with
DBS. The goggles have a sensor that activates an LCD
screen to show patients a tile pattern on the floor. The checkerboard pattern
projected on the floor makes them feel like they have something to step over.
There is also an auditory feedback cue. The goggles are still being tested.
About 10 patients have tried them so far in this study, and the goal is 20
people. Patients in the study wore the goggles for a half hour a day for two
weeks, and some seem to still see the tiles after they take off the goggles. For
more information go to the
The goggles have a sensor that activates an LCD
screen to show patients a tile pattern on the floor. The checkerboard pattern
projected on the floor makes them feel like they have something to step over.
There is also an auditory feedback cue. The goggles are still being tested.
About 10 patients have tried them so far in this study, and the goal is 20
people. Patients in the study wore the goggles for a half hour a day for two
weeks, and some seem to still see the tiles after they take off the goggles. For
more information go to the  Those most common for rotigotine were
nausea (17%), dyskinesia (12%), somnolence (12%), and application-site erythema/pruritus
(9%). The work was carried out for Schwarz Pharma, who are developing
transdermal rotigotine. For more information go to the
Those most common for rotigotine were
nausea (17%), dyskinesia (12%), somnolence (12%), and application-site erythema/pruritus
(9%). The work was carried out for Schwarz Pharma, who are developing
transdermal rotigotine. For more information go to the  It decreased the dopamine turnover rate by
40%, and inhibited dopamine transporter and uptake. 3-OMD induced cytotoxic
effects via oxidative stress and decreased mitochondrial membrane potential,
indicating that it can also damage neuronal cells. 3-OMD also potentiated L-dopa
toxicity. Therefore, L-dopa treatment can accelerate the progression of
Parkinson's Disease because of its metabolite 3-O-Methyldopa.
It decreased the dopamine turnover rate by
40%, and inhibited dopamine transporter and uptake. 3-OMD induced cytotoxic
effects via oxidative stress and decreased mitochondrial membrane potential,
indicating that it can also damage neuronal cells. 3-OMD also potentiated L-dopa
toxicity. Therefore, L-dopa treatment can accelerate the progression of
Parkinson's Disease because of its metabolite 3-O-Methyldopa. Several
prospective studies have assessed the association between coffee consumption and
Parkinson's disease risk, but the results were inconsistent. A massive study was
carried out in Finland on nearly 30,000 subjects. They examined the association
of coffee and tea consumption with the risk of Parkinson's Disease. There
was a clear trend in both men and women for coffee drinkers to be less prone to
Parkinson's Disease. In both sexes combined, the multivariate-adjusted
likelihood for subjects drinking 3 or more cups of tea per day compared to
non-tea drinkers also showed a clearly lower risk of Parkinson's Disease.
Several
prospective studies have assessed the association between coffee consumption and
Parkinson's disease risk, but the results were inconsistent. A massive study was
carried out in Finland on nearly 30,000 subjects. They examined the association
of coffee and tea consumption with the risk of Parkinson's Disease. There
was a clear trend in both men and women for coffee drinkers to be less prone to
Parkinson's Disease. In both sexes combined, the multivariate-adjusted
likelihood for subjects drinking 3 or more cups of tea per day compared to
non-tea drinkers also showed a clearly lower risk of Parkinson's Disease. Biopharmaceutical company
BrainStorm Cell
Therapeutics has arranged a meeting
with the U.S. Food and Drug Administration (FDA) to coordinate clinical trials
for its flagship product for the treatment of Parkinson's disease. The company
said it aims to be the first worldwide to win approval to conduct trials using
adult stem cells taken from patients. BrainStorm has hired Dr Andra E.Miller,
director of cell and gene therapies of the Biologics Consulting Group Inc. to
handle the FDA negotiations.
For more information go to the
Biopharmaceutical company
BrainStorm Cell
Therapeutics has arranged a meeting
with the U.S. Food and Drug Administration (FDA) to coordinate clinical trials
for its flagship product for the treatment of Parkinson's disease. The company
said it aims to be the first worldwide to win approval to conduct trials using
adult stem cells taken from patients. BrainStorm has hired Dr Andra E.Miller,
director of cell and gene therapies of the Biologics Consulting Group Inc. to
handle the FDA negotiations.
For more information go to the  They
said their analysis showed that the lower doses of safinamide
alongside dopamine led to improvements and reduced the need to make changes to
treatment regimes. "Additional Phase III studies are planned to further assess
the efficacy of this dose of safinamide," they added. For more information
go to the
They
said their analysis showed that the lower doses of safinamide
alongside dopamine led to improvements and reduced the need to make changes to
treatment regimes. "Additional Phase III studies are planned to further assess
the efficacy of this dose of safinamide," they added. For more information
go to the  In an independent trial in England, continuous GDNF infusion in five patients
showed good tolerance, few side effects and clinical benefit within 3 months of
treatment. The clinical improvement was sustained and progressive. A
second clinical trial in ten patients also showed a greater than 30% bilateral
benefit in both on and off-medication score at 24 weeks. A randomized controlled
clinical trial was recently conducted to confirm the previous clinical trial
results. However, GDNF infusion over 6 months did not confer the predetermined
level of clinical benefit. The researchers suggest that technical differences
between the recent trial and previous studies contributed to this negative
outcome.
In an independent trial in England, continuous GDNF infusion in five patients
showed good tolerance, few side effects and clinical benefit within 3 months of
treatment. The clinical improvement was sustained and progressive. A
second clinical trial in ten patients also showed a greater than 30% bilateral
benefit in both on and off-medication score at 24 weeks. A randomized controlled
clinical trial was recently conducted to confirm the previous clinical trial
results. However, GDNF infusion over 6 months did not confer the predetermined
level of clinical benefit. The researchers suggest that technical differences
between the recent trial and previous studies contributed to this negative
outcome. They found that pergolide and
cabergolide increase the risk of a heart valve condition by 7 and 5 times, when
compared to another Parkinson's Disease drug. Patients on Parkinson's drugs
besides these did not increase their risk of developing the condition.
Permax had already been withdrawn from sale in the U.S.A..
For full details go to the
They found that pergolide and
cabergolide increase the risk of a heart valve condition by 7 and 5 times, when
compared to another Parkinson's Disease drug. Patients on Parkinson's drugs
besides these did not increase their risk of developing the condition.
Permax had already been withdrawn from sale in the U.S.A..
For full details go to the


 The
two volumes on Parkinsons disease in the Handbook of Clinical Neurology
cover the dramatic advances in understanding of the biochemical background of
Parkinsonism and the resulting developments in its pharmacological management.
These volumes give an account of the subject for both clinical neurologists and
those researching in the neurosciences. Part I covers the scientific background,
general aspects of Parkinson's disease, clinical aspects and etiology. Part II
covers the medical and surgical treatment of Parkinsons disease, complications
of therapy and the other parkinsonian syndromes.
For details of Part I go to
The
two volumes on Parkinsons disease in the Handbook of Clinical Neurology
cover the dramatic advances in understanding of the biochemical background of
Parkinsonism and the resulting developments in its pharmacological management.
These volumes give an account of the subject for both clinical neurologists and
those researching in the neurosciences. Part I covers the scientific background,
general aspects of Parkinson's disease, clinical aspects and etiology. Part II
covers the medical and surgical treatment of Parkinsons disease, complications
of therapy and the other parkinsonian syndromes.
For details of Part I go to
 They suggest
that it's possible that simultaneously treating both the dopamine and
norepinephrine loss could further ameliorate Parkinson's disease.
For full details go to the
They suggest
that it's possible that simultaneously treating both the dopamine and
norepinephrine loss could further ameliorate Parkinson's disease.
For full details go to the
 However, there is only a general tendency for Parkinson's Disease
to be associated with uric acid levels. Uric acid levels could therefore not
determine whether or not somebody had or would have Parkinson's Disease. Also,
the reason for this association is likely to be different from the explanation
given. High protein diets, likely to produce uric acid, are also high in the
L-dopa precursors L-tyrosine and L-phenylalanine. L-tyrosine and
L-phenylalanine would lessen the tendency towards Parkisnon's Disease because
they can increase dopamine levels. For more information on
However, there is only a general tendency for Parkinson's Disease
to be associated with uric acid levels. Uric acid levels could therefore not
determine whether or not somebody had or would have Parkinson's Disease. Also,
the reason for this association is likely to be different from the explanation
given. High protein diets, likely to produce uric acid, are also high in the
L-dopa precursors L-tyrosine and L-phenylalanine. L-tyrosine and
L-phenylalanine would lessen the tendency towards Parkisnon's Disease because
they can increase dopamine levels. For more information on
 The Michael J. Fox Foundation for
Parkinson's Research announced $1.2 million in awards to four research teams working
in the drug development pipeline. The research projects will be :
the autophagy pathway, a cellular pathway involved
in clearing away protein such as alpha-synuclein; optimizing administration of
the compound MX-4565, a non-feminizing estrogen analog that has been shown to be
effective in protecting nerve cells from toxic stresses; the potential of the
protein osteopontin as a Parkinson's Disease treatment; and a molecule called
fisetin, which can maintain levels of glutathione, which is produced naturally
as a defence against oxidative stress.
The Michael J. Fox Foundation for
Parkinson's Research announced $1.2 million in awards to four research teams working
in the drug development pipeline. The research projects will be :
the autophagy pathway, a cellular pathway involved
in clearing away protein such as alpha-synuclein; optimizing administration of
the compound MX-4565, a non-feminizing estrogen analog that has been shown to be
effective in protecting nerve cells from toxic stresses; the potential of the
protein osteopontin as a Parkinson's Disease treatment; and a molecule called
fisetin, which can maintain levels of glutathione, which is produced naturally
as a defence against oxidative stress.  The drug
being tested is significantly different from over-the-counter creatine
supplements. Efficacy will be determined over 5 to 7 years by means of tests
that measure ability to walk, carry out other daily activities, cognitive
function, and general quality of life. For full details go to the
The drug
being tested is significantly different from over-the-counter creatine
supplements. Efficacy will be determined over 5 to 7 years by means of tests
that measure ability to walk, carry out other daily activities, cognitive
function, and general quality of life. For full details go to the
 The U.S. Food and Drug Administration (FDA) has
approved a new higher dose strength of Stalevo (carbidopa, levodopa and
entacapone). Stalevo is indicated for Parkinson's disease patients with signs
and symptoms of end-of-dose "wearing off." The approval of the higher 200mg
Stalevo (50 mg carbidopa, 200 mg levodopa, 200 mg entacapone) gives physicians
greater dosing flexibility in the treatment of patients experiencing symptom
re-emergence due to end of dose wearing off. The new dosage strength may lessen
the burden of managing multiple medications
The U.S. Food and Drug Administration (FDA) has
approved a new higher dose strength of Stalevo (carbidopa, levodopa and
entacapone). Stalevo is indicated for Parkinson's disease patients with signs
and symptoms of end-of-dose "wearing off." The approval of the higher 200mg
Stalevo (50 mg carbidopa, 200 mg levodopa, 200 mg entacapone) gives physicians
greater dosing flexibility in the treatment of patients experiencing symptom
re-emergence due to end of dose wearing off. The new dosage strength may lessen
the burden of managing multiple medications A
survey of over 6000 Parkinson's disease patients were examined for the
relationship between freezing and age, gender, duration, subjective severity of
Parkinson's disease, and antiparkinsonian medication. 47% of people with
Parkinson's Disease reported experiencing freezing regularly. Freezing was
associated with longer disease duration and the more advanced stages of
Parkinson's Disease. Freezing episodes were more likely in men than in women.
Freezing was more common in patients taking, in addition to L-dopa, Entacapone,
Amantadine, or dopamine agonists. Patients considering tremor as their main
symptom reported freezing less frequently. Common Parkinson's Disease drugs
given in combination with L-dopa were not negatively related to episodes of
freezing.
A
survey of over 6000 Parkinson's disease patients were examined for the
relationship between freezing and age, gender, duration, subjective severity of
Parkinson's disease, and antiparkinsonian medication. 47% of people with
Parkinson's Disease reported experiencing freezing regularly. Freezing was
associated with longer disease duration and the more advanced stages of
Parkinson's Disease. Freezing episodes were more likely in men than in women.
Freezing was more common in patients taking, in addition to L-dopa, Entacapone,
Amantadine, or dopamine agonists. Patients considering tremor as their main
symptom reported freezing less frequently. Common Parkinson's Disease drugs
given in combination with L-dopa were not negatively related to episodes of
freezing.
 There was significant
improvement in UPDRS (the major method of Parkinson's Disease assessment)
compared with the baseline. Serum dopamine level also was significantly elevated
over the same interval. There was a significant correlation between UPDRS and
serum dopamine level before and after treatment. Therefore, improved motor
performance in PD after repeated session of rTMS may be related to an elevation
of serum dopamine concentration.
There was significant
improvement in UPDRS (the major method of Parkinson's Disease assessment)
compared with the baseline. Serum dopamine level also was significantly elevated
over the same interval. There was a significant correlation between UPDRS and
serum dopamine level before and after treatment. Therefore, improved motor
performance in PD after repeated session of rTMS may be related to an elevation
of serum dopamine concentration. 
