.gif) VIARTIS
|
||||||||||||
|
PARKINSON'S DISEASE NEWS |
||||||||||||
|
|
Parkinson's Disease News covers all significant new research, reports, books, and resources concerning Parkinson's Disease. Articles are chosen on the basis of their medical significance or potential interest. Our overwhelming priority is the facts, regardless of whether they contradict prevailing views or vested interests. Analysis and further information are provided either to explain the background or implications, or to balance misleading claims. If you notice errors or inadequacies, or dispute what is written, or want to propose articles, please e-mail [email protected].
8th September 2014 - News release SUBCUTANEOUS LIQUID L-DOPA FOR PARKINSON'S DISEASE
NeuroDerm have announced that the first patients with severe Parkinson's
Disease have been enrolled and dosed in a Phase IIa trial of ND0612H.
ND0612H is a high-dose form of liquid L-dopa and carbidopa (which is the
same as Sinemet) but which is delivered continuously through subcutaneous
administration (via the skin) by a belt-pump. Unlike the most comparable
methods of administering L-dopa, no surgery at all is needed.
|
|
||||||||||
.gif) |
||||||||||||
| �2006-2014 Viartis | ||||||||||||
| 2014-09-08 19:44:26 | ||||||||||||
| [email protected] | ||||||||||||








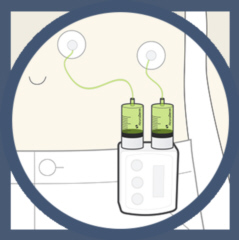 ND0612L
is the low dose drug form intended for moderate Parkinson's Disease. ND0612L
has just completed patient enrolment and treatment in a Phase II
double-blind, randomised, placebo-controlled study. ND0612L was shown in
previous phase I and phase IIa studies to be safe and tolerable, reaching
steady state, clinically meaningful L-dopa blood concentrations.
ND0612L
is the low dose drug form intended for moderate Parkinson's Disease. ND0612L
has just completed patient enrolment and treatment in a Phase II
double-blind, randomised, placebo-controlled study. ND0612L was shown in
previous phase I and phase IIa studies to be safe and tolerable, reaching
steady state, clinically meaningful L-dopa blood concentrations. E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail
E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail
