.gif) VIARTIS
|
|||||
|
PARKINSON'S DISEASE NEWS |
|||||
|
|
18th April 2012 - News release LEVODOPA-CARBIDOPA INTESTINAL GEL CLINICAL TRIAL RESULTS
Participants had had Parkinson's Disease for an average of over 10 years, and experienced an average of over 6 hours "off" time. Their average "off" time decreased by 4 hours per day with LCIG, which was 1.9 fewer hours of "off" time compared to levodopa-carbidopa IR in tablet form. Adverse events occurred in 95% of people on LCIG, and 100% of people on levodopa-carbidopa IR tablets. The most common adverse events were complication of device insertion (51%), abdominal pain (42%), procedural pain (32%), nausea (25%), constipation (21%), orthostatic hypotension (18%), post-operative wound infection (17%), and incision site erythema (16%). Treatment-related serious adverse events were reported in 14% of the LCIG patients, and 21% of the levodopa-carbidopa IR tablet patients. For more information go to the News release. For a printable version of this article click here. For more current news go to Parkinson's Disease News.
|
|
|||
|
Parkinson's Disease News details all significant new research, news reports, new books, and new resources concerning Parkinson's Disease and those medical disorders that often coincide with Parkinson's Disease. It is compiled from an analysis of all newly published research, news reports, new clinical trials, all newly published books, and new web sites. A summary and analysis of the new research are provided, as well as links to the complete abstracts and news reports
|
|||||
.gif) |
|||||
| �2006-2012 Viartis | |||||
| 2015-09-04 03:05:12 | |||||
| [email protected] | |||||
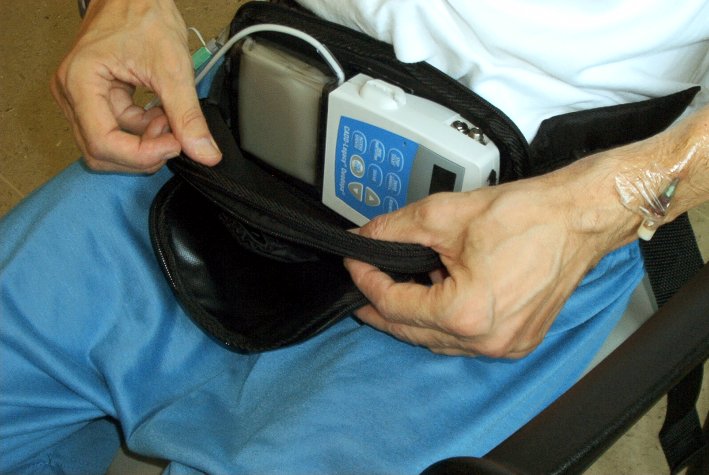 Abbott�s
have announced the results of the Phase 3 clinical trial in the U.S.A. of their
investigational treatment for advanced Parkinson�s Disease. The treatment
involved levodopa-carbidopa intestinal gel (LCIG). The treatment is already
approved in many countries outside the U.S.A. as Duodopa. LCIG contains the same
active medication as Sinemet, which is levodopa-carbidopa IR tablets. LCIG was
administered using a procedurally-implanted tube connected to a portable pump
that delivers the medication directly into the small intestine, where it is
absorbed into the bloodstream, providing a continuous delivery of medication
during the 16 hours a day of pump use.
Abbott�s
have announced the results of the Phase 3 clinical trial in the U.S.A. of their
investigational treatment for advanced Parkinson�s Disease. The treatment
involved levodopa-carbidopa intestinal gel (LCIG). The treatment is already
approved in many countries outside the U.S.A. as Duodopa. LCIG contains the same
active medication as Sinemet, which is levodopa-carbidopa IR tablets. LCIG was
administered using a procedurally-implanted tube connected to a portable pump
that delivers the medication directly into the small intestine, where it is
absorbed into the bloodstream, providing a continuous delivery of medication
during the 16 hours a day of pump use.  E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail
E-MAIL NOTIFICATION : If you would like to be
notified by e-mail when any new articles are added to Parkinson's Disease News, please merely
e-mail